2157
Changing Cortical Myeloarchitecture and Not Morphology Are Associated with Early Cognitive Development1Bill & Melinda Gates Foundation, Seattle, WA, United States, 2Advanced Baby Imaging Lab, Rhode Island Hospital, Providence, RI, United States, 3Pediatrics, Rhode Island Hospital, Providence, RI, United States
Synopsis
Early brain development is punctuated by rapid changes in the white and gray matter, including myelination and advancing microstructure, architecture, volume, and geometry. Here we explored the relationships between, and influence of, these changing neuroanatomical properties and advancing cognitive skill in healthy and typically developing toddlers and young children. Unlike past results in older children and adults, we find few significant associations between cortical morphometry measures and cognition. Instead, we find strong associations between intra-cortical myelin content and measures of language and visual reception function.
Introduction
Neuroimaging studies of child brain development often focus on measures of white matter development (myelination) and cortical morphology, two fundamental and complementary developmental processes. Both myelination and cortical maturation are intricately connected, responsive to environmental influence, and associated with emerging brain function, cognition, and behavioral skills; and alterations in each have been associated with developmental disorders, as well as learning and intellectual disorders. While prior work has documented associations between cortical thickness, surface area, and cognitive performance, recent work has suggested these cortical measures may be directly influenced by the cortical myeloarchitecture, which may also contribute to observed functional differences. In this work, we used longitudinal neuroimaging measures of cortical morphometry and myelin content to resolve to independent and combined influence on emerging cognitive skills in healthy children between 1 and 5 years of age.Methods
447 neuroimaging datasets from 307 (137 females) children were drawn from a large accelerated-longitudinal study of healthy brain development(1). Data were acquired on a Siemens 3T Trio with a 12-channel head RF array with children under 4 years of age imaged during non-sedated sleep and older children whilst watching a movie. Children were swaddled with a MedVac immobilizer and foam cushions to minimize motion, and scanned with acoustically derated sequences. mcDESPOT(2) was used to estimate myelin water fraction (MWF)(3) and quantify the myeloarchitecture, while conventional T1w MP-RAGE data was used quantify brain anatomy and cortical structure, including cortical thickness (CT), surface area (SA), and curvature and volume via FreeSurfer(4). Following cortical analysis, cortical regions were superimposed onto the subject’s MWF image and mean cortical MWF values calculated.Cognitive ability and verbal and non-verbal functioning was assessed in all children using the Mullen Scales of Early Learning (MSEL(5)). A series of analyses were performed using this complement of data to investigate the relationship between changing MWF, CT, SA, volume, and curvature, and individual level changing fine motor, visual reception, and expressive and receptive language skill.
We quantified individual measures of structural and functional change using a series of linear mixed-effect models, i.e., for myelination and CT in each region,
MWFi,j=β0,j+β1,jlog(age),
CTi,j=β0,j+β1,j(age),
and for cognition (fine motor, FM, as example)
FMi,j=β0,j+β1,j(age)
The correlation between the individual change estimates (β1,j) were then calculated in each cortical region and between each imaging metric and cognitive measure pair.
Results & Discussion
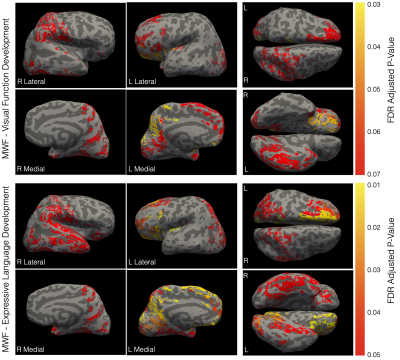
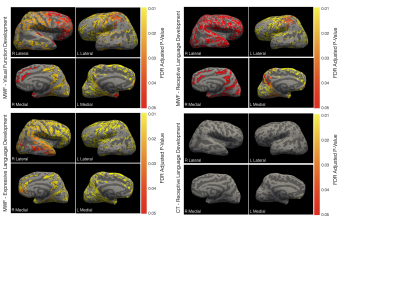
We found no significant associations between developing cortical morphology measure and emerging cognitive score, we did find significant correlations between individual rates of myelination and expressive language development throughout the brain, including (Fig. 1) conventional language i.e., bilateral temporal and orbitofrontal lobes, pars ortbitalis, and pars opercularis, as well as non-language areas, i.e., right precuneous, bilateral paracentral gyrus, insula, right postcentral, right lingual gyrus, fusiform gyrus, and portions of the cingulate cortex. With respect to visual functioning, we found borderline significant (adjusted p < 0.06) in occipital, frontal, and temporal regions, including bilateral paracentral gyrus, insula, orbitofrontal gyrus, right fusiform gyrus, and right lingual gyrus.While structural MRI studies have traditionally been cross-sectional in nature, with emphasis on either cortical or white matter development, longitudinal multimodal studies have increasingly opened up the possibility of interrogating the relationships between these interconnected processes and their change with child age. Throughout much of the cortex, we found that intra-cortical myelin content was a significant predictor of cortical thickness but not other morphometric properties. This agrees with other past work investigating changes in cortical myelination and morphology in older children and adult visual cortex. In prior longitudinal cortical development studies, rates of cortical thinning through late childhood and adolescence have been associated with later general cognitive outcomes (intelligence coefficient) and developmental disorder(6, 7). In infants, early cortical thickness and surface area have been associated with later language, motor, and visual skill, but with relatively weak contributions over and above environmental factors such as maternal education(8).
Extending this work, we explored the direct relationships between rates of cortical thinning (as well as surface area expansion, volume growth, and curvature development) and concurrent rates of functional development across the motor, language, and visual reception domains from 1 to ~6 years of age. We found no significant associations when controlling for maternal education. However, in contrast, significant relationships were observed between rate of cortical myelination and cognitive development across the cortex with respect to expressive language and, at p<0.06, visual reception (Fig. 2).
Further confirming the role of intracortical myelin to function, we find that when all measures are combined in the same predictive model, we find only significant associations between MWF and language (expressive and receptive) and visual processing; apart from left hemisphere medial orbitofrontal and rostral anterior cingulate cortical thickness with respect to receptive language.
These results shed new light on the importance of cortical myelination in early child development, a critical process that can be modified by nutrition, caregiving quality, and environmental stimulation, and may have broad and important implications for understanding early neurodevelopment and how early therapeutic interventions may impact functional outcomes.
Acknowledgements
No acknowledgement found.References
1. Deoni SC, Dean DC, 3rd, O'Muircheartaigh J, Dirks H, Jerskey BA. Investigating white matter development in infancy and early childhood using myelin water faction and relaxation time mapping. Neuroimage. Nov 15 2012;63(3):1038-53. doi:10.1016/j.neuroimage.2012.07.037
2. Deoni SC, Rutt BK, Arun T, Pierpaoli C, Jones DK. Gleaning multicomponent T1 and T2 information from steady-state imaging data. Magn Reson Med. Dec 2008;60(6):1372-87. doi:10.1002/mrm.21704
3. MacKay AL, Laule C. Magnetic Resonance of Myelin Water: An in vivo Marker for Myelin. Brain Plast. Dec 21 2016;2(1):71-91. doi:10.3233/BPL-160033
4. Fischl B. FreeSurfer. Neuroimage. Aug 15 2012;62(2):774-81. doi:10.1016/j.neuroimage.2012.01.021
5. E.M. M. Mullen Scales of Early Learning. American Guidance Services, Inc; 1995.
6. Shaw P, Greenstein D, Lerch J, et al. Intellectual ability and cortical development in children and adolescents. Nature. Mar 30 2006;440(7084):676-9. doi:10.1038/nature04513
7. Shaw P, Lalonde F, Lepage C, et al. Development of cortical asymmetry in typically developing children and its disruption in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. Aug 2009;66(8):888-96. doi:10.1001/archgenpsychiatry.2009.103
8. Girault JB, Cornea E, Goldman BD, et al. Cortical Structure and Cognition in Infants and Toddlers. Cereb Cortex. Mar 21 2020;30(2):786-800. doi:10.1093/cercor/bhz126
Figures