2129
Phase correction in liver single-shot DW-EPI acquired with partial Fourier encoding1Department of Diagnostic and Interventional Radiology, School of Medicine, Technical University of Munich, Munich, Germany, 2Philips Healthcare, Best, Netherlands, 3Philips Healthcare, Hamburg, Germany
Synopsis
In single shot diffusion imaging of moving organs, undesired motion during diffusion encoding introduces rapid phase variations in the obtained images. When partial Fourier encoding is used to reduce SNR loss in short T2 tissues, the motion-induced rapid phase variation compromises the partial Fourier reconstruction, resulting in “worm-like” artifacts in the reconstructed images. This work shows that by applying phase correction before the partial Fourier reconstruction motion-induced artifacts can be mitigated, improving the quality of diffusion-weighted images in liver applications.
Purpose
With the capabilities of depicting pathology-dependent changes in molecular diffusion of water in tissues, diffusion imaging possesses great clinical value both in brain and abdominal applications (1,2). The nature of diffusion imaging is the encoding of molecular motion, which makes undesired tissue motion such as cardiac pulsation, respiratory motion, and patient motion the major confounding factor (3-5). Undesired motion during diffusion encoding introduces rapid phase variations in the obtained images. When partial Fourier encoding is used to reduce SNR loss due to short T2 in applications such as liver DWI, the motion-induced rapid phase variation compromises the partial Fourier reconstruction, resulting in “worm-like” artifacts in the reconstructed images (6,7). By applying phase correction before the partial Fourier reconstruction, this work shows that motion-induced artifacts can be mitigated, improving the quality of diffusion-weighted images in liver applications.Methods
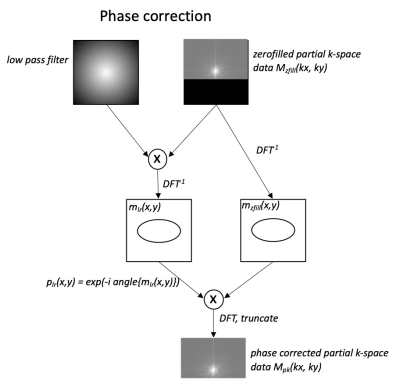
Phase correctionFigure 1 describes the phase correction step before the partial Fourier reconstruction. The motion-induced phase is estimated from the filtered, symmetrically acquired k-space and subtracted from the image reconstructed from the zero-filled k-space. The phase corrected image is transformed back to k-space domain where the resulted k-space data is truncated to yield phase corrected partial Fourier k-space data. Any partial Fourier reconstruction algorithms can then be used to reconstruct the final image.
MR measurements
Two healthy volunteers were scanned on a 3T whole-body scanner (Ingenia Elition, Philips Healthcare) using the 16-channel torso coil and the built-in-table 12-channel posterior coil. A spin echo diffusion-weighted sequence in combination with a single-shot EPI readout was employed with the following acquisition parameters: FOV = 420 x 370 mm2, voxel size = 3 x 3 x 6 mm3, 10 slices with 6 mm gap, b-values = 0, 200, 400, 600 s/mm2 with the number of averages of 4, 5, 6, 8, respectively, 3 diffusion encoding directions (b-value > 0). All scans were respiratory triggered with a fixed trigger delay of 300 ms. Data with both full Fourier encoding and partial Fourier encoding factor (pF) of 0.7 were acquired. Pulsed gradient spin echo (PGSE) and asymmetric velocity compensated (asym vc) diffusion encoding waveforms were experimented with TE (pF = 0.7)/TE (full) = 46/59 ms and 73/86 ms, respectively (8).
Results
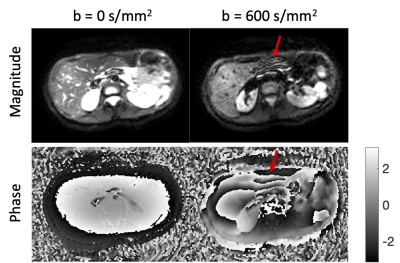
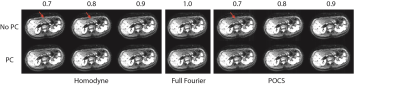
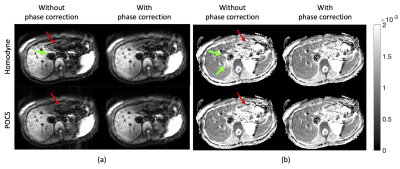
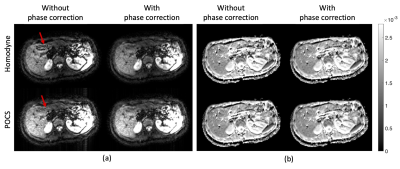
Figure 2 presents the phases of typical b = 0 and b = 600 s/mm2 images. The rapid phase variation, especially in the left liver lobe of the b = 600 s/mm2 image violated the smooth phase assumption of partial Fourier reconstruction algorithms leading to compromised reconstructed images. Figure 3 shows the simulated partial Fourier data at pFs of 0.7, 0.8, and 0.9 from the acquired PGSE full Fourier data. When no phase correction was done (no PC) and at low pFs, “worm-like” artifacts can be observed in the left lobe of the liver, where significant motion, driven by cardiac motion, occurred. As the pF increased, the “worm-like” artifact appearance reduced. Regarding the partial Fourier reconstruction algorithm, POCS was less affected by the artifacts than homodyne (9,10). Employing the phase correction procedure before partial Fourier reconstruction removed the previously observed artifacts both in homodyne and POCS reconstructed images. Figure 4 shows the PGSE partial Fourier encoding data with pF = 0.7. Without phase correction both homodyne and POCS result in DWI with “worm-like” artifacts in the left liver lobe and for the case of homodyne also around the inferior vena cava (IVC) (Figure 4a). Phase correction removes the artifacts and restores image quality (Figure 4a). Corresponding ADC maps show an overestimation of ADC around the IVC when homodyne is used without phase correction (Figure 4b). In the left liver lobe both homodyne and POCS without phase correction yield noisier ADC values than with phase correction (Figure 4b). With the asym vc waveform, “worm-like” artifacts were still visible in the reconstructed image without phase correction (Figure 5a). However, no significant difference was observed between the ADC estimation from DWI with and without phase correction.Discussion and conclusion
Partial Fourier reconstruction algorithms rely on the assumption that the image phase is smooth. In diffusion imaging of moving organs such as the liver, the rapidly varying motion-induced phase causes the violation of the above assumption and therefore compromises the reconstructed images. With the PGSE waveform, by estimation and correction of the motion-induced phase before the partial Fourier reconstruction artifact-free DWIs and correct ADC maps can be obtained. When the motion-compensated encoding waveform (asym vc) was used, “worm-like” artifacts were visible in few of the DWIs and the estimated ADC maps were not significantly affected by the artifacts. The less frequent appearance of the “worm-like” artifacts and more correct ADC maps even without phase correction with asym vc waveform is expected since motion compensation results in less variation in motion-induced phases.In conclusion, when partial Fourier encoding is employed in diffusion imaging of moving organs phase correction should be performed before the partial Fourier reconstruction to eliminate motion-induced artifacts.Acknowledgements
The present work was supported by Philips Healthcare.References
1. Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology 1986;161(2):401-407.
2. Taouli B, Koh DM. Diffusion-weighted MR imaging of the liver. Radiology 2010;254(1):47-66.
3. Kwee TC, Takahara T, Niwa T, Ivancevic MK, Herigault G, Van Cauteren M, Luijten PR. Influence of cardiac motion on diffusion-weighted magnetic resonance imaging of the liver. MAGMA 2009;22(5):319-325.
4. Liau J, Lee J, Schroeder ME, Sirlin CB, Bydder M. Cardiac motion in diffusion-weighted MRI of the liver: artifact and a method of correction. J Magn Reson Imaging 2012;35(2):318-327.
5. Murphy P, Wolfson T, Gamst A, Sirlin C, Bydder M. Error model for reduction of cardiac and respiratory motion effects in quantitative liver DW-MRI. Magn Reson Med 2013;70(5):1460-1469.
6. Holdsworth SJ, Skare S, Bammer R. On the application of phase correction and use of k-space entropy in partial Fourier diffusion-weighted EPI. 17th Annual Meeting of ISMRM. Honolulu, HI 2009. p 4617.
7. Storey P, Frigo FJ, Hinks RS, Mock BJ, Collick BD, Baker N, Marmurek J, Graham SJ. Partial k-space reconstruction in single-shot diffusion-weighted echo-planar imaging. Magn Reson Med 2007;57(3):614-619.
8. Mc Tavish S, Van AT, Peeters JM, Ogino T, Hock A, Rummeny E, Braren R, Karampinos DC. Partial velocity-compensated optimized diffusion encoding for combined motion compensation and residual vessel signal suppression in liver ADC mapping. 27th Annual Meeting of ISMRM. Montreal, Canada 2019. p 1709.
9. Liang ZP, Boada FE, Constable RT, Haacke E, Lauterbur PC, Smith MR. Constrained reconstruction methods in MR imaging. Reviews of Magnetic Resonance in Medicine 1992;4:67-185.
10. Noll DC, Nishimura DG, Macovski A. Homodyne detection in magnetic resonance imaging. IEEE Trans Med Imaging 1991;10(2):154-163.
Figures