2126
Reproducibility and Variability of Liver ADC Using Simultaneous Multi-slice DWI with Different Breathing Schemes and Different MR Vendors1West China Hospital, Sichuan University, Chengdu, China, 2MR collaborations, Siemens Healthcare Ltd., Shanghai, China, 3MR Research, GE Healthcare, Beijing, China
Synopsis
Diffusion-weighted imaging (DWI) provides anatomic and functional information, and apparent diffusion coefficient (ADC) has been proposed as a valuable biomarker in liver diseases. Simultaneous multi-slice (SMS) technique allows for exciting and acquiring multiple slices at the same time, and thus reducing scan time, which has been recently proved to be feasible in liver imaging. Our study investigated the reproducibility and variability of liver ADC of SMS-DWI with different breathing schemes and different vendors. We found good reproducibility of liver ADC across different MR vendors in both breath-hold and free-breathing manner. However, liver ADC is less reproducible between different breathing schemes.
Introduction
Diffusion-weighted imaging (DWI), which provides both anatomic information and functional metrics, has been widely investigated and applied in diffuse and focal liver diseases in the past two decades1. Despite its considerable diagnostic value, the relatively long acquisition time of DWI is a limiting factor in clinical practice, and the quantitative parameter - apparent diffusion coefficient (ADC) - can be interfered by breathing and cardiac motion artifacts2. Simultaneous multi-slice (SMS) technique allows for exciting multiple slices at the same time, and thus reducing the scan time3. Previous studies have validated the feasibility of SMS-DWI in abdominal imaging, which showed improved image quality and sufficient signal to noise ratio when compared to conventional DWI4-6. As a valuable quantitative biomarker for the potential widespread use, one major prerequisite of ADC is the reproducibility of measurements, especially in longitudinal and multicenter studies. However, whether liver ADC of SMS-DWI obtained with different breathing techniques and different MR platforms is reproducible remains an open question.Purpose
To investigate the reproducibility and variability of liver ADC values derived from SMS-DWI with breath-hold and free-breathing techniques from two different MR vendors.Materials and Methods
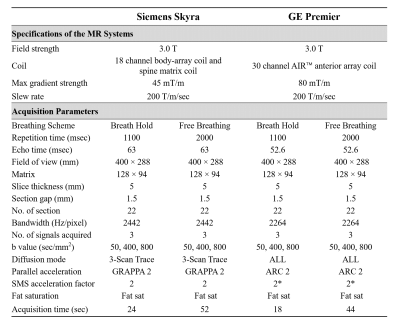
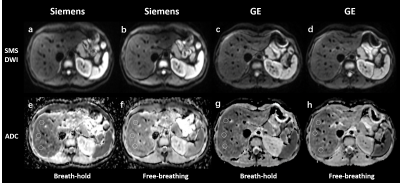
Seventeen healthy volunteers (seven men and ten women; mean age ± standard deviation [SD], 32.5 years ± 13.4) were enrolled between October 2020 and December 2020. Breath-hold and free-breathing SMS-DWI were performed in each subject by using 3.0 T MR systems from two different vendors (Vendor 1: Siemens Healthcare; and Vendor 2: GE Healthcare). The acquisition parameters were kept identical between two MR systems to the extent possible (Figure 1), and the minimum available echo time was chosen in both MR systems. By using mono-exponential fitting of all b values, ADC maps were automatically generated on each MR system's console. Two experienced readers independently reviewed the four series of images and performed regions of interest (ROI) analysis in a blind fashion. Three circle ROIs with an average area of 1.5 cm2 were positioned in the right liver lobe at similar slice positions between scans, avoiding major vessels and artifacts (Figure 2). Inter-reader agreement of ADC values was assessed by calculating intraclass correlation coefficient (ICC). Difference of mean ADC between two breathing schemes and two vendors was evaluated with t test or Wilcoxon signed rank test, where appropriate. The coefficient of variation (CV, equal to SD divided by mean) was calculated for each breathing scheme and vendor.Results
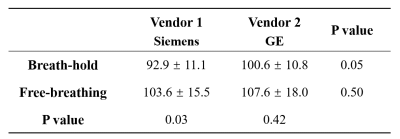
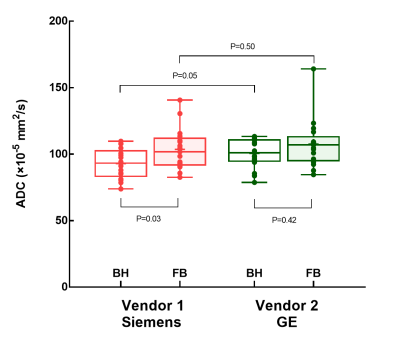
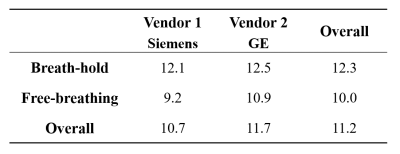
Inter-reader agreement of liver ADC was excellent in SMS-DWI, with ICCs ranging from 0.80 to 0.93. The liver ADC values of breath-hold and free-breathing SMS-DWI were (92.9 ± 11.1) ×10-5 mm2/s and (103.6 ± 15.5) ×10-5 mm2/s in vendor 1; and (100.6 ± 10.8) ×10-5 mm2/s and (107.6 ± 18.0) ×10-5 mm2/s in vendor 2 (Figure 3 and Figure 4). In vendor 1, the liver ADC of free-breathing SMS-DWI was significantly higher than that of breath-hold SMS-DWI (P=0.03). In vendor 2, no significant difference was found in ADC values from different breathing schemes (P=0.42). The liver ADC measurements from two vendors did not show significant difference in both breathing schemes (P=0.05 and P=0.50). CVs ranged from 9.2% to 12.5% depending on the breathing scheme and vendor, with the ADC of free-breathing SMS-DWI in vendor 1 showing the lowest CV, and the ADC of breath-hold SMS-DWI in vendor 2 showing the highest CV (Figure 5). The mean CV for liver ADC measurement was 11.2%.Discussion
In current study, liver ADC was found to be slightly higher in vendor 2 than that of vendor 1 in both breath-hold and free-breathing SMS-DWI, but not in a significant statistical way. A previous study by Donati et al. also reported the similar results7, indicating that the liver ADC measurements derived from conventional DWI were slightly different across different vendors. Together with our findings, liver ADC of SMS-DWI seems reproducible in liver imaging across different vendors, which may be used as a reliable biomarker in the follow-up and multicenter studies. In addition, we found large CV in liver ADC values of breath-hold SMS-DWI, compared to that of free-breathing manner, which was in good agreement with previous findings that breathing schemes influence liver ADC measurements and their reproducibility in conventional DWI6,8. The tentative explanation might be the motion artifact due to insufficient breath-hold ability, since we set the scanning time to 18 to 24 seconds in breath-hold SMS-DWI to cover enough b values.Conclusion
There is no significant differences of liver ADC across different MR vendors in both breath-hold and free-breathing SMS-DWI. However, liver ADC of SMS-DWI is less reproducible between different breathing schemes, with breath-hold technique showing more variations.Acknowledgements
None.References
1. Kele PG, van der Jagt EJ. Diffusion weighted imaging in the liver. World J Gastroenterol. 2010;16(13):1567-1576.
2. Mazaheri Y, Do RK, Shukla-Dave A, et al. Motion correction of multi-b-value diffusion-weighted imaging in the liver. Acad Radiol. 2012;19(12):1573-1580.
3. Taron J, Weiß J, Martirosian P, et al. Clinical Robustness of Accelerated and Optimized Abdominal Diffusion-Weighted Imaging. Invest Radiol. 2017 Oct;52(10):590-595.
4. Taron J, Martirosian P, Erb M, et al. Simultaneous multislice diffusion-weighted MRI of the liver: Analysis of different breathing schemes in comparison to standard sequences. J Magn Reson Imaging. 2016 Oct;44(4):865-79.
5. Tavakoli A, Attenberger UI, Budjan J, et al. Improved Liver Diffusion-Weighted Imaging at 3 T Using Respiratory Triggering in Combination With Simultaneous Multislice Acceleration. Invest Radiol. 2019 Dec;54(12):744-751.
6. Pei Y, Xie S, Li W, et al. Evaluation of simultaneous-multislice diffusion-weighted imaging of liver at 3.0 T with different breathing schemes. Abdom Radiol (NY). 2020 Nov;45(11):3716-3729.
7. Donati OF, Chong D, Nanz D, et al. Diffusion-weighted MR imaging of upper abdominal organs: field strength and intervendor variability of apparent diffusion coefficients. Radiology. 2014 Feb;270(2):454-63.
8. Chen X, Qin L, Pan D, et al. Liver diffusion-weighted MR imaging: reproducibility comparison of ADC measurements obtained with multiple breath-hold, free-breathing, respiratory-triggered, and navigator-triggered techniques. Radiology. 2014 Apr;271(1):113-25.
Figures