2123
Motion-Robust, High-SNR Diffusion MRI of the Liver using Optimized Gradient Waveforms and Deep Learning Reconstruction1Medical Physics, University of Wisconsin Madison, Madison, WI, United States, 2Radiology, University of Wisconsin Madison, Madison, WI, United States, 3GE Healthcare, Boston, MA, United States, 4GE Healthcare, Waukesha, WI, United States
Synopsis
Diffusion MRI of the liver has important applications in the detection of metastatic lesions. Unfortunately, conventional diffusion MRI is highly sensitive to cardiac-induced motion in the left lobe, whereas recently developed motion-robust diffusion MRI methods lead to lower SNR. This work demonstrates the feasibility of combining motion-robust diffusion MRI with DL reconstruction for improved DWI of the liver, including high motion robustness and high SNR. In early-stage evaluation, the combined technique provides excellent motion robustness, quantitative reliability, and high SNR throughout the entire liver, and may have application in the detection and evaluation of metastatic liver lesions.
Introduction
Diffusion MRI of the liver has important applications in the detection of metastatic lesions, and may also play a role in the assessment of diffuse liver disease (eg: fibrosis) [1,2]. Unfortunately, conventional diffusion MRI is highly sensitive to cardiac-induced motion in the left lobe [3-5], which leads to signal dropouts in diffusion-weighted images (DWI) and bias and variability in quantitative metrics such as the apparent diffusion coefficient (ADC). These artifacts may complicate the detection and evaluation of lesions throughout the entire liver, as required in clinical practice, or the assessment of fibrosis. Recently developed optimized motion-robust diffusion gradient waveforms have been shown to overcome the motion-induced signal dropouts, demonstrating superior reliability compared to conventional monopolar diffusion waveforms [6-8]. Even though these motion-robust waveforms are designed to minimize the achievable echo time (TE), they nevertheless result in TE increases compared to conventional monopolar acquisitions. Given the short T2 of the liver (eg: <40ms), this TE increase can lead to substantial SNR loss, which may complicate the detection of small lesions. In recent years, deep learning (DL) based methods have demonstrated outstanding potential for denoising of low-SNR data. Our main hypothesis is that the combination of optimized motion-robust DWI acquisition with a denoising DL reconstruction [9] will enable motion-robust, high-SNR DWI of the liver. This work evaluates the feasibility of motion-robust DWI of the liver with DL reconstruction.Methods
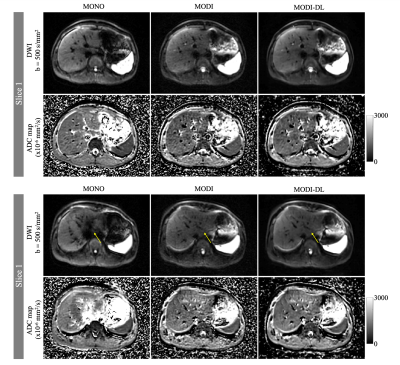
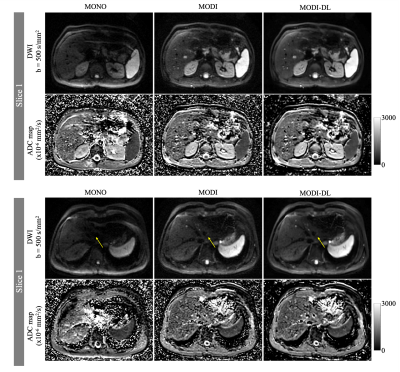
MRI Acquisition: Two healthy volunteers were scanned at 3T (Signa Premier, GE Healthcare), under IRB approval and informed written consent. For each volunteer, two breath-held DWI acquisitions were obtained with b-values of 50 and 500 s/mm2 (see Table 1 for parameters), including a conventional DWI with monopolar gradients (MONO, TE=44ms), and a motion-robust DWI using M1-optimized Diffusion Imaging (MODI) gradient waveforms (TE=66ms) [8].DL Reconstruction: The diffusion raw data from MODI acquisition was then reconstructed in two ways, first using the standard echo-planar reconstruction and second using a DL Recon algorithm based on a Convolutional Neural Network (CNN) trained to optimize SNR. For this second series, images were reconstructed with a tunable noise reduction factor set to 75%.
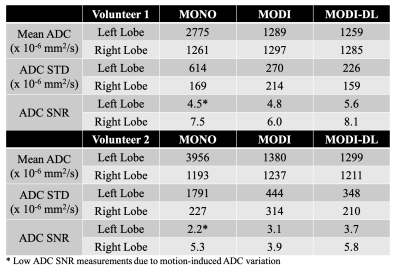
Assessment of Motion-Robustness and SNR: In order to evaluate the motion-robustness of the three DWI reconstructions (monopolar, as well as MODI with and without DL reconstruction), ADC measurements were performed in the two regions of interest (ROIs) within the right and left liver lobe, respectively, for comparison across lobes. This approach is based on the assumption that motion-robust ADC is similar across lobes in healthy volunteers. Further, as a surrogate measure of SNR, the standard deviation of ADC within each of the ROIs described above was also measured.
Results
Figures 1-2 show example DW images and ADC maps in the two volunteers. Conventional monopolar DWI resulted in adequate SNR in the right lobe of the liver (eg: moderate ADC standard deviation in the right lobe), but severe shading and signal dropouts in the left lobe. These signal dropouts lead to substantial ADC bias in the left lobe, with ADC>2500x10-6mm2/s compared to ~1200x10-6mm2/s in the right lobe. In contrast, MODI DWI demonstrated excellent motion-robustness without signal dropouts, and with consistent ADC throughout the liver (1200-1400x10-6mm2/s across both lobes). However, MODI DWI had lower SNR with a relatively high ADC standard deviation (Table 2). By combining MODI with the DL reconstruction, MODI-DL DWI maintained the motion-robustness throughout the liver without signal dropouts. Further, MODI-DL also maintained the consistent ADC throughout the liver (ADC of 1200-1300x10-6mm2/s across both lobes). Finally, MODI-DL led to substantial increases in apparent SNR compared to MODI, as reflected by the reduced ADC standard deviation in both liver lobes, thus providing SNR comparable to that of monopolar DWI. Table 2 summarizes these quantitative measurements.Discussion
This work has demonstrated the feasibility of combining recently developed optimized diffusion gradient waveforms with DL reconstruction for improved DWI of the liver with high motion robustness and high SNR. Upon further validation, this approach may enable DWI with reliable whole-liver coverage (including the left lobe), as needed in clinical practice.Importantly, our results suggest that DL reconstruction is able to improve SNR in MODI DWI, without introducing artifacts and without decreasing the quantitative reliability of MODI. This approach may even open the door to accelerated DWI of the liver with shorter acquisitions, which would have substantial implications for clinical imaging and workflows.
These preliminary results need to be validated in a larger cohort, including healthy volunteers and patients with known or suspected metastatic lesions. This further validation remains as future work.
Conclusion
Combining motion-robust diffusion MRI with denoising deep learning reconstruction has the potential to achieve high motion robustness and high SNR DWI in the liver.Acknowledgements
Support for this research was provided by the University of Wisconsin - Madison Office of the Vice Chancellor for Research and Graduate Education with funding from the Wisconsin Alumni Research Foundation, as well as from the UW Departments of Radiology and Medical Physics. The authors would also like to acknowledge research support from GE Healthcare and Bracco Diagnostics to the University of Wisconsin-Madison.References
[1] Lewis, S., Dyvorne, H., Cui, Y. and Taouli, B., 2014. Diffusion-weighted imaging of the liver: techniques and applications. Magnetic Resonance Imaging Clinics, 22(3), pp.373-395.
[2] Annet, L., Peeters, F., Abarca‐Quinones, J., Leclercq, I., Moulin, P. and Van Beers, B.E., 2007. Assessment of diffusion‐weighted MR imaging in liver fibrosis. Journal of Magnetic Resonance Imaging: An Official Journal of the International Society for Magnetic Resonance in Medicine, 25(1), pp.122-128.
[3] Naganawa, S., Kawai, H., Fukatsu, H., Sakurai, Y., Aoki, I., Miura, S., Mimura, T., Kanazawa, H. and Ishigati, T., 2005. Diffusion-weighted imaging of the liver: technical challenges and prospects for the future. Magnetic Resonance in Medical Sciences, 4(4), pp.175-186.
[4] Murphy, P., Wolfson, T., Gamst, A., Sirlin, C. and Bydder, M., 2013. Error model for reduction of cardiac and respiratory motion effects in quantitative liver DW‐MRI. Magnetic resonance in medicine, 70(5), pp.1460-1469.
[5] Kwee, T.C., Takahara, T., Niwa, T., Ivancevic, M.K., Herigault, G., Van Cauteren, M. and Luijten, P.R., 2009. Influence of cardiac motion on diffusion-weighted magnetic resonance imaging of the liver. Magnetic Resonance Materials in Physics, Biology and Medicine, 22(5), pp.319-325.
[6]Peña‐Nogales, Ó., Zhang, Y., Wang, X., de Luis‐Garcia, R., Aja‐Fernández, S., Holmes, J. H., & Hernando, D. (2019). Optimized Diffusion‐Weighting Gradient Waveform Design (ODGD) formulation for motion compensation and concomitant gradient nulling. Magnetic resonance in medicine, 81(2), 989-1003.
[7]Aliotta, E., Wu, H.H. and Ennis, D.B., 2017. Convex optimized diffusion encoding (CODE) gradient waveforms for minimum echo time and bulk motion–compensated diffusion‐weighted MRI. Magnetic resonance in medicine, 77(2), pp.717-729.
[8] Zhang Y, Peña‐Nogales Ó, Holmes JH, Hernando D. Motion‐robust and blood‐suppressed M1‐optimized diffusion MR imaging of the liver. Magnetic resonance in medicine. 2019 Jul;82(1):302-11.
[9] Lebel, R. M. (2020). Performance characterization of a novel deep learning-based MR image reconstruction pipeline. arXiv preprint arXiv:2008.06559.
Figures