2121
Microvascular Invasion has no Independent Effect on Recurrence in Small Hepatocellular Carcinoma: A Propensity Score Matching Analysis1Shanghai Institute of Medical Imaging, Shanghai, China, 2Department of Radiology, Zhongshan Hospital, Fudan University, Shanghai, China, 3Department of Liver Surgery, Liver Cancer Institute, Zhongshan Hospital, Fudan University, Shanghai, China, 4Key Laboratory of Carcinogenesis and Cancer Invasion of Ministry of Education, Shanghai, China, 5Department of Medical Imaging, Shanghai Medical College, Fudan University, Shanghai, China
Synopsis
Whether microvascular invasion (MVI) is a prognosis factor for small hepatocellular carcinoma (sHCC) is controversial, a preoperatively predictive model based on gadoxetate disodium (Gd-EOB-DTPA) MRI is clinical needed for MVI in sHCC.We concluded that alpha-fetoprotein>20 ng/mL, non-smooth margin, incomplete or absent capsule, peritumoral enhancement and larger tumor size were independent risk factors for MVI in patients with sHCC ≤3 cm. Although MVI independently impaired RFS before propensity score matching (PSM), it was ultimately identified as a potential but not an independent risk factor for recurrence in sHCC patients after PSM balancing the confounder — tumor size.
Introduction
Increasing evidence shows that microvascular invasion (MVI) impairs the surgical outcomes 1,2 of hepatocellular carcinoma (HCC) patients, and tumor size is closely correlated to the incidence of MVI 2 and poor prognosis 3. This implies the tumor size may be a potential confounder — major threat to the validity of the retrospective study, for MVI in predicting outcomes. Meanwhile, an earlier diagnosis of MVI in HCC patients, especially for small HCC (sHCC), will provide the clinicians a better understanding of pathobiological behaviours for optimizing therapy initiation and improving long-term survivals. While the definition of sHCC varies greatly by a confusing criteria from 2 cm to 5 cm in the maximum tumor diameter 4-8, recent studies have indicated that tumor size growing larger than 3 cm is a critical turning point towards more aggressive behaviour 5,7,8. Compared with extracellular agents, gadoxetate disodium (Gd-EOB-DTPA) with hepatocyte-specific properties not only reflects the vascularity of HCC lesions, but alsooffers a favourable identifiability of sHCC or early HCC 9-11. However, previous studies concerning MVI in HCC patients are retrospective analysis and selective bias cannot be avoided. Hence, this study aimed to evaluate the preoperative value of Gd-EOB-DTPA MRI for predicting MVI, and implement a propensity score matching (PSM) to investigate the correlation between MVI and recurrence-free survival in patients with sHCC ≤3 cm.Methods
Between March 2012 and September 2020, 455 consecutive patients with pathologically confirmed HCC ≤3 cm who underwent preoperative Gd-EOB-DTPA MRI were retrospectively enrolled. Uni- and multivariate logistic regression combined with cox regression were conducted to find the confounding factors in the cohort. Propensity score matching (PSM) was employed to balance the biases between MVI and non-MVI groups. Nomogram with C-index visualized the MVI predictive model.Results
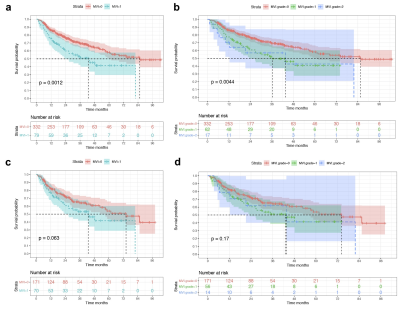
Before PSM, histologic MVI independently affected tumor recurrence (hazard ratio: 1.555, 95% confidence interval: 1.055-2.293, P = 0.026), but highly biased between MVI-positive and MVI-negative groups regarding the confounder of tumor size (propensity score: 0.249 ± 0.105 Vs. 0.179 ± 0.106, P <0.001). Meanwhile, the frequency of MVI significantly increased as tumor size growing (from 0 to 12.6 cm, P <0.001). After PSM, 70 of 79 MVI cases matched with 171 non-MVI (total 332), and no biases were observed between the two groups (propensity score: 0.238 ± 0.104 Vs. 0.217 ± 0.109, P = 0.186). The median recurrence time was longer in non-MVI than MVI group (74.3 months Vs 43.0 months, P = 0.063), however, MVI failed to be an independent prognosis factor for RFS in sHCC. Further, multivariate logistic regression identified 5 characteristics (AFP, tumor size, tumor margin, peritumoral enhancement, radiologic capsule) which were markedly associated with MVI in sHCC and incorporated into the nomogram with excellent predictive performance in training (AUC/C-index: 0.884/0.874), validation (AUC/C-index: 0.845/0.828) and test cohorts(AUC/C-index: 0.903/0.954).Discussion
Elevated AFP level 12,13, non-smooth margin 1,2,13, the incomplete/absent capsule enhancement 1,12, larger tumor size 12,14 and peritumoral enhancement 1,2,13 have been reported to be independent risk factors for MVI, which were also highly applicable to our sHCC patients. Notably, peritumoral enhancement, capsule and margin statuses imply peritumoral biological behaviour, reflecting an aggressive tendency to invade the tumor capsule and infiltrate into the peritumoral non-neoplastic parenchyma 15. Pathologically, peritumoral parenchyma is representative of tumor heterogenetity and rich in highly invasive cells 8, where is the first and most frequently vulnerable to MVI 15,16. Controversially, MVI exerts an ambiguous effect on sHCC outcome. A non-PSM study of Du et al. 4 presented that MVI independently shorted progression-free and overall survival, and increasing tumor size facilitated higher incidences of MVI in solitary sHCC ≤3 cm. The results were consensus on our findings before PSM. However, the conclusion of Du et al. neglected the biases of respective study and the potential confounder (e.g., tumor size) for MVI in prognostic analyses. At the state of the art, the ratio of radical treatment in patients with sHCC ≤3 cm has markedly increased, posing an urgent and practical issue in hepatic surgery 7. Hence, we speculate that this random controlled trial (RCT)-like PSM study allows clinicians more time to optimize therapeutic schedule 1) with a reasonable surgical margin, for the reason that MVI status is independently irrespective of recurrence in sHCC ≤3 cm; 2) with a reasonable residual liver volume, for reducing the frequency of hepatic dysfunction and infection and improving the safety of liver resection 17.Conclusion
Mainly based on the peritumoral hallmarks of Gd-EOB-DTPA MRI, our preoperative nomogram could excellently distinguish the histologic MVI status. However, MVI was not an independent risk factor for recurrence in patients with sHCC ≤3 cm after PSM balancing the confounder — tumor size.Acknowledgements
Funding: Supported by National Natural Science Foundation of China (No.91859107), Shanghai Science and Technology Committee (No.19411965500), Shanghai Municipal Key Clinical Specialty (No.W2019-018), Clinical Research Plan of SHDC (No.SHDC2020CR1029B).References
1. Xu, X., et al.Radiomic analysis of contrast-enhanced CT predicts microvascular invasion and outcome in hepatocellular carcinoma. J Hepatol70, 1133-1144 (2019).
2. Lee, S., Kim, S.H., Lee, J.E., Sinn, D.H. & Park, C.K. Preoperative gadoxetic acid-enhanced MRI for predicting microvascular invasion in patients with single hepatocellular carcinoma. J Hepatol67, 526-534 (2017).
3. Takuma, Y., et al.Nomograms to Predict the Disease-free Survival and Overall Survival after Radiofrequency Ablation for Hepatocellular Carcinoma. Intern Med57, 457-468 (2018).
4. Du, M., et al.Microvascular invasion (MVI) is a poorer prognostic predictor for small hepatocellular carcinoma. BMC Cancer14, 38 (2014).
5. Lu, X.Y., et al.Pathobiological features of small hepatocellular carcinoma: correlation between tumor size and biological behavior. J Cancer Res Clin Oncol137, 567-575 (2011).6. Lim, C., et al.Above 5 cm, size does not matter anymore in patients with hepatocellular carcinoma. World journal of surgery38, 2910-2918 (2014).
7. Cong, W.M. & Wu, M.C. Small hepatocellular carcinoma: current and future approaches. Hepatology international7, 805-812 (2013).
8. Cong, W.M., et al.Practice guidelines for the pathological diagnosis of primary liver cancer: 2015 update. World J Gastroenterol22, 9279-9287 (2016).
9. Renzulli, M., et al.New hallmark of hepatocellular carcinoma, early hepatocellular carcinoma and high-grade dysplastic nodules on Gd-EOB-DTPA MRI in patients with cirrhosis: a new diagnostic algorithm. Gut67, 1674-1682 (2018).
10. Kitao, A., et al.Gadoxetic acid-enhanced MR imaging for hepatocellular carcinoma: molecular and genetic background. Eur Radiol30, 3438-3447 (2020).11. Cho, E.S. & Choi, J.Y. MRI features of hepatocellular carcinoma related to biologic behavior. Korean J Radiol16, 449-464 (2015).
12. Lei, Z., et al.Nomogram for Preoperative Estimation of Microvascular Invasion Risk in Hepatitis B Virus-Related Hepatocellular Carcinoma Within the Milan Criteria. JAMA Surg151, 356-363 (2016).
13. Yang, L., et al.A Radiomics Nomogram for Preoperative Prediction of Microvascular Invasion in Hepatocellular Carcinoma. Liver Cancer8, 373-386 (2019).
14. Banerjee, S., et al.A computed tomography radiogenomic biomarker predicts microvascular invasion and clinical outcomes in hepatocellular carcinoma. Hepatology62, 792-800 (2015).
15. Hu, H., et al.A non-smooth tumor margin on preoperative imaging assesses microvascular invasion of hepatocellular carcinoma: A systematic review and meta-analysis. Sci Rep7, 15375 (2017).
16. Hu, H.T., et al.Peritumoral tissue on preoperative imaging reveals microvascular invasion in hepatocellular carcinoma: a systematic review and meta-analysis. Abdom Radiol (NY)43, 3324-3330 (2018).
17. Schindl, M.J., Redhead, D.N., Fearon, K.C., Garden, O.J. & Wigmore, S.J. The value of residual liver volume as a predictor of hepatic dysfunction and infection after major liver resection. Gut54, 289-296 (2005).
Figures