2120
Influence of portal vein occlusion on portal flow and liver elasticity in an animal model1ICube, UMR 7357 CNRS, University of Strasbourg, Strasbourg, France, 2IHU-Strasbourg, Institute of image-guided surgery, Strasbourg, France, 3Interventional Neuroradiology Department, University Hospitals of Strasbourg, Strasbourg, France, 4Siemens Healthcare SAS, Saint Denis, France, 5Siemens Medical Solutions USA, Inc., Chicago, IL, United States, 6Public Healthcare Department, University Hospitals Strasbourg, Strasbourg, France
Synopsis
Hepatic fibrosis causes an increase in the liver stiffness, a parameter measured by elastography and widely used as diagnosis method. This study aims at determining the extent to which a portal occlusion due to concomitant portal vein thrombosis can also affect these mechanical properties. Portal vein occlusion was generated by progressive inflations of a balloon catheter in the portal vein of four swines. The portal flow and liver stiffness were investigated using 4D-flow MRI and MR-Elastography. This vascular mechanism is shown to be sufficient to attenuate the increase in stiffness due to moderate fibrosis and may lead to false-negative diagnosis.
Objectives
Hepatic fibrosis causes an increase in liver stiffness, a parameter measured by elastography and widely used for diagnosis [1,2]. The concomitant presence of portal vein thrombosis (PVT) implies a change in hepatic portal inflow that could also affect liver elasticity [3]. Despite good agreement between elasticity measurement and fibrosis stage, a recent case study has reported PVT as a potential source of false-negative diagnosis of fibrosis using elastography [4]. The main objective of this study is to determine the extent to which the presence of portal occlusion can affect the mechanical properties of the liver through portal flow modifications and potentially lead to misdiagnosis of fibrosis and hepatic cirrhosis by elastography.Material and Methods
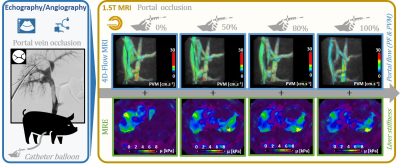
Portal vein occlusion was generated by percutaneous insertion and inflation of a balloon catheter in an intrahepatic branch of the portal vein of four anesthetized swines (authorization APAFIS #14092-2018031513247711v1) under ultrasound (Acuson S3000, Siemens Healthcare) and X-ray fluoroscopy guidance (Artis Zeego system, Siemens Healthcare) (figure 1). Once the catheter balloon was in place, MRI acquisitions were conducted in a 1.5T MRI scanner (MAGNETOM Aera, Siemens Healthcare) using the 18-channel body matrix surface coil and the spine coil, with the animal lying in supine position. Saline was used for progressive balloon inflation (0, 50, 80 and 100% portal vein occlusion). The portal flow parameters, namely peak flow (PF) and peak velocity magnitude (PVM), and liver mechanical properties (shear modulus) were then investigated using the 4D-flow prototype (TR/TE = 51.68/3.77 ms, transit time TT = 20 ms to 440 ms by 52.5 ms steps, FOV 284 mm x 414 mm, matrix 106 x 192, 20 slices, slice thickness 2.5 mm, 11 time frames, encoding velocity 50 cm.s-1 in all directions) and MR Elastography (MRE) (commercial pneumatic surface exciter Resoundant®, frequency 60 Hz, TR/TE = 50/23.75 ms, FOV 239 mm x 300 mm, matrix 204 x 256, 3 slices, slice thickness 5 mm), respectively, for progressive obstructions of the portal vein (0, 50, 80 and 100%). High-resolution images are also acquired using prototypical SPIRAL VIBE sequence (TR/TE = 4.57/0.05 ms, FOV 300 mm x 300 mm, matrix 288 x 288, 176 slice, slice thickness 1 mm) to measure the intraluminal sectional vein obstruction.Results
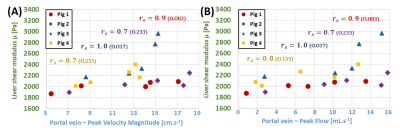
Baseline PVM, PF and liver’s shear modulus were measured at initial 0% occlusion as 15.5 ± 2.5 cm.s-1, 13.5 ± 1.6 mL.s-1 and 2,268 ± 131 Pa, respectively, averaged over the four subjects. Experimental results indicate that the reduction of the intrahepatic venous blood flow (PF/PVM decrease of 29.3%/8.5%, 51.0%/32.3% and 83.3%/53.6%, respectively) measured with 50%, 80% and 100% obstruction of the portal vein section results in a decrease of liver stiffness by 0.8 ± 0.1%, 7.7 ± 0.4% and 12.3 ± 0.9% compared to the reference no-occlusion values, respectively. Despite the small number of subjects, the concordance of the MRE measurement was good, with a concordance correlation coefficient general value of 0.890 (0.6577; 0.9679). A decrease of liver’s shear modulus with portal PF and PVM were observed (figure 2). As a control, PF and PVM were also quantified in the inferior vena cava. These control values did not seem to be influenced by the portal occlusion.Discussion and Conclusions
Results showed a decrease of the liver’s shear modulus correlated with the reduction in portal flow, as observed in case of PVT. Considering the orders of magnitude of the stiffness variations, this study confirms that vascular effects due to the frequent presence of PVT concomitantly with an acute fibrotic stage or cirrhosis (F4 METAVIR grade) are not likely to alter the diagnosis of the latter by liver elastography. Conversely, moderate fibrosis (F2-F3 METAVIR grades) causes only a slight increase in liver stiffness that may be attenuated in the presence of PVT by the described vascular effects. Although the occurrence of concomitant PVT at moderate stages of fibrosis remains low [5], there is a risk of false-negative results in the diagnosis of moderate fibrosis using elastography, as recently suggested in a clinical case study [4]. The vascular implications of PVT on liver elasticity reported in this study are therefore of interest in optimizing the diagnosis of moderate hepatic fibrosis with elastography.Acknowledgements
This study was funded by the ANR (Agence Nationale de la Recherche) within the Investissements d’Avenir program for the IHU Strasbourg (Institute of image-guided surgery, ANR-10-IAHU-02). The authors would like to thank Dr. Thomas Benkert (Siemens Healthcare GmbH, Erlangen, Germany) for providing the prototype SPIRAL VIBE sequence as well as imaging platform of the IHU-Strasbourg and the IRIS platform of ICube.References
[1] Huwart L, Peeters F, Sinkus R, Annet L, Salameh N, ter Beek LC, Horsmans Y, Van Beers BE. Liver fibrosis: Non-invasive assessment with MR elastography. NMR Biomed. 2006;19(2):173-179.
[2] Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008;48(5):835-847.
[3] Ipek-Ugay S, Tzschätzsch H, Braun J, Fischer T, Sack I. Physiologic reduction of hepatic venous blood flow by the Valsalva maneuver decreases liver stiffness. J Ultrasound Med. 2017;36(7):1305-1311.
[4] Huang R, Gao ZH, Tang A, Sebastiani G, Deschenes M. Transient elastography is an unreliable marker of liver fibrosis in patients with portal vein thrombosis. Hepatology. 2018;68(2):783-785.
[5] Papatheodoridis GV, Papakonstantinou E, Andrioti E, Cholongitas E, Petraki K, Kontopoulou I, Hadziyannis SJ. Thrombotic risk factors and extent of liver fibrosis in chronic viral hepatitis. Gut. 2003;52(3):404-409.
Figures