2115
Application of APT and DKI in predicting the postoperative liver decompensation and recurrence of early HCC after TACE treatment1The First Affiliated Hospital of Xinxiang Medical University, Xinxiang, China, 2GE Healthcare, MR Research China, Beijing, China
Synopsis
In this study we assessed the feasibility of quantitative parameters derived from amide proton transfer (APT) and diffusion kurtosis imaging (DKI) in predicting the response to transcatheter arterial chemoembolization (TACE) treatment for hypervascular hepatocellular carcinoma (HCC). The combination model of quantitative parameters of APT and DKI showed good diagnostic performance in predicting the recurrence and postoperative liver decompensation. Therefore, APT and DKI can be used as potential imaging biomarkers to predict the effect of early-stage HCC after TACE treatment.
Introduction
Hepatocellular carcinoma (HCC) has become the second most common cancer and the sixth most common cause of cancer-related death worldwide[1]. Most patients lose indications for surgical resectionare in their initial visit. Transarterial chemoembolization(TACE) is the standard treatment for patients with intermediate-stage HCC. However, the tumor recurrence rate remains high after curative resection and the prognosis is still poor. Therefore, it is essential to predict the response to TACE for HCC. DKI and APT can reflect the characteristics of cell density, microcirculation perfusion, and tissue complexity of lesions[2-4], which have been previously applied to evaluate the diagnosis, grading and therapeutic efficacy. However, the role of APT and DKI in predicting the postoperative liver decompensation and the recurrence of early-stage HCC treated with TACE has not been reported yet. This study is aimed to investigate value of APT and DKI imaging in predicting the postoperative liver decompensation and the recurrence of early stage HCC treated with TACE.Methods
In this study, 63 patients with HCC were recruited. All the subjects underwent abdomen MRI on a 3T MR scanner (Discovery MR750W, GE Healthcare, USA) with a 32-channel phased-array torso coil. MR scans included routine abdomen sequences, APT (TR, 3000 ms; TE, 12.0 ms; FOV, 36×36 cm2; matrix, 128×128; layer thickness, 5 mm; RF, 2.0 μT; saturation time, 500 ms, and 1 NEX resulting in 52 images) and DKI. The DKI data were acquired along 30 directions, with b values of 0, 1000, and 2000 s/mm2. Two radiologists, who were blinded to the pathologic results, did image analyses on GE AW4.6 Workstation. The ROIs were manually placed on the maximal section of each lesion, carefully avoiding the area of cystic degeneration, necrosis, and bleeding. At last, APT parameter MTRasym and the DKI parameters including mean diffusivity (MD), and mean kurtosis (MK) were measured. Liver function was reviewed one week after TACE. The recurrence rate of HCC treated with TACE was recorded after a median follow-up of 6 months. The subjects were divided into the recurrence and non-recurrence groups, postoperative hepatic decompensation group and non-postoperative hepatic decompensation groups. Quantitative variables were presented as mean ± standard deviation and compared by using Student's t test or the Wilcoxon Mann-Whitney test (in the event of a skewed distribution). Univariate and multivariate logistic regression analyses were used to identify independent factors for tumor response to TACE in the response group. Diagnostic performance was evaluated with the operating characteristic (ROC) curve. The optimal threshold of each parameter and the corresponding sensitivity and specificity were calculated. P < 0.05 represents statistical significance.Results and Discussion
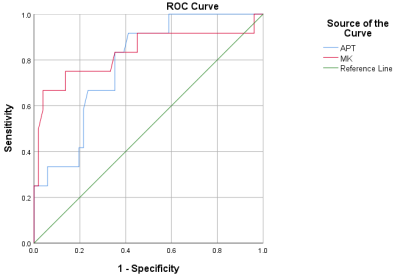
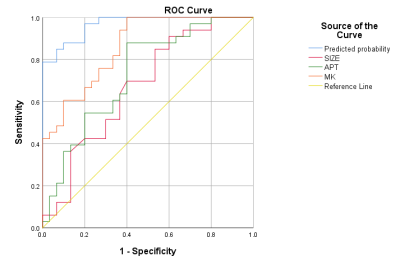
The MK (0.79 ± 0.06) and MTRasym (1.62±0.21) value of the postoperative hepatic insufficiency group in Fig. 1 was higher than the MK (0.66±0.11) and APT value (1.34±0.29) value of the non-postoperative hepatic insufficiency group in Fig. 2 with P < 0.05. MK value (AUC = 0.881, cutoff value = 0.628) showed the higher AUC than MTRasym value (AUC= 0.783, cutoff value = 0.505) in predicting the liver decompensation of early stage HCC treated with TACE.The MTRasym and MK value of the recurrence group in Fig.1 was higher than that of the non-recurrence group in Fig. 2 (P < 0.05). There were no significant differences in MD values between the two groups (P > 0.05). Under multivariate analysis, tumor size (P = 0.035), MTRasym (P = 0.017), and MK (P = 0.011) of tumor tissue were identified as independent predictors for tumor response to TACE. The AUC of tumor size, MK, MTRasym and the prediction model of tumor size+MK+MTRasym were 0.673, 0.861, 0.728 and 0.967, respectively.
Conclusion
This study showed that the postoperative hepatic insufficiency group and the recurrence group of HCC treated with TACE had higher MTRasym value and MK value. APT combined with DKI imaging is useful in predicting the postoperative hepatic insufficiency and recurrence of early stage HCC treated with TACE.Acknowledgements
This study was supported by the National Natural Science Foundation of China (81971595 and 81771812)References
[1].Zhang Z, Jiang H, Chen J, et al. Hepatocellular carcinoma: radiomics nomogram on gadoxetic acid-enhanced MR imaging for early postoperative recurrence prediction. Cancer Imaging. 2019;19(1):22. Published 2019 May 14.
[2].Goshima S, Kanematsu M, Noda Y, Kondo H, Watanabe H, Bae KT. Diffusion kurtosis imaging to assess response to treatment in hypervascular hepatocellular carcinoma. AJR Am J Roentgenol. 2015;204(5):W543-W549.
[3].Payabvash S. Quantitative diffusion magnetic resonance imaging in head and neck tumors. Quant Imaging Med Surg. 2018;8(10):1052-1065. doi:10.21037/qims.2018.10.14
[4].Chen T, Li Y, Lu SS, et al. Quantitative evaluation of diffusion-kurtosis imaging for grading endometrial carcinoma: a comparative study with diffusion-weighted imaging. Clin Radiol. 2017;72(11):995.e11-995.e20.
[5].Yoshimaru D, Takatsu Y, Suzuki Y, et al. Diffusion kurtosis imaging in the assessment of liver function: Its potential as an effective predictor of liver function. Br J Radiol. 2019;92(1094):20170608.
Figures
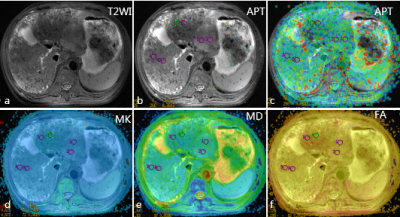
Figure 1. Images of the postoperative hepatic insufficiency and recurrence 6 months after TACE in 65-year-old male liver cancer patients
a-c: T2WI map and amide proton transfer (APT) map overlaid on MR image with tumor boundary outlined; d-e: Related parameters of diffusion kurtosis imaging.
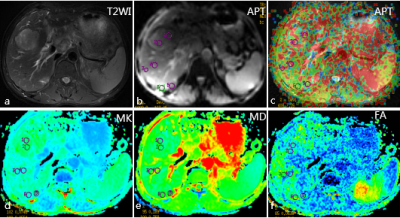
Figure 2. Images of the non-postoperative hepatic insufficiency and non-recurrence 6 months after TACE in 58-year-old male liver cancer patients
a-c: T2WI map and amide proton transfer (APT) map overlaid on MR image with tumor boundary outlined; d-e: Related parameters of diffusion kurtosis imaging.