2113
Data-driven modification of the LI-RADS major feature system: toward better sensitivity and simplicity1Radiology, West China Hospital, Sichuan University, Chengdu, China, 2Radiology, Duke University Medical Center, Durham, NC, United States, 3Radiation Oncology, Duke University School of Medicine, Durham, NC, United States, 4Radiology, University of Ottawa, Ottawa, ON, Canada, 5Epidemiology, University of Ottawa, Ottawa, ON, Canada, 6Center for Advanced Magnetic Resonance in Medicine, Duke University Medical Center, Durham, NC, United States, 7Division of Gastroenterology, Department of Medicine, Duke University Medical Center, Durham, NC, United States
Synopsis
We aimed to propose a revised LI-RADS (rLI-RADS) for hepatocellular carcinoma (HCC) based on the LI-RADS version 2018 (v2018) major feature system on gadoxetate disodium (EOB)-enhanced MRI. We retrieved 224 consecutive at-risk patients with 742 LR-3 to LR-5 hepatic observations from a prospectively-collected database. In the training set, LI-RADS v2018 major features evaluated by three independent radiologists were used to develop rLI-RADS according to the likelihood of HCC. Compared with LI-RADS v2018, rLI-RADS was completely data-driven, remarkably simpler, and demonstrated significantly optimized diagnostic sensitivity and accuracy while maintaining reasonable specificity for HCC.
Body of the Abstract
IntroductionLiver Imaging Reporting and Data System (LI-RADS) was developed to standardize diagnosis and reporting for liver observations in patients at-risk for hepatocellular carcinoma (HCC). Despite accepted as a reliable diagnostic system with over 90% specificity1, the application of LI-RADS is hampered by substantial complexity2 and suboptimal diagnostic sensitivity1,3. Previous works have explored possible solutions to address these issues4-9. However, many of them were based on investigator experiences, and limited evidences are available regarding the performances of the many available combinations of imaging features. In addition, although there are strong data supporting the LI-RADS major features as the main determinants of HCC diagnosis1,10,11, the assignment of a lesion to a given LI-RADS risk stratum (LR-3 to LR-5) has not been rigorously explored on large patient samples.
Therefore, in a data-driven manner, we aimed to assess the risk of HCC in lesions with a variety of combinations of LI-RADS major features and propose revisions to the LI-RADS diagnostic table (rLI-RADS) on gadoxetate disodium (EOB)-enhanced MRI to improve simplicity and sensitivity while maintaining high specificity.
Methods
Consecutive at-risk patients with LR-3 to LR-5 observations were analyzed retrospectively from a prospective observational registry collected between July 2015 to September 2018 [Clinical trial registration No: ChiCTR1900026668].
In the training set, LI-RADS version 2018 (v2018) major features evaluated by three independent abdominal radiologists were used to develop rLI-RADS. Specifically, the LI-RADS v2018 CT/MRI diagnostic table was first converted into a decision tree, and the proportion of HCC (%HCC, equivalent to positive predictive value) of each leaf node was computed. Based on the associated %HCC, the rLI-RADS categories (rLR) were then defined as the following: 1) leaf nodes with %HCC ≥ 90% were categorized as rLR-5; 2) those with %HCC < 20% as rLR-3; 3) and those with %HCC between 20% and 90% as rLR-4. On a per-lesion basis, a composite reference standard according to either pathology or imaging was established.
In an independent testing set, diagnostic performances were computed using a generalized estimating equation model to account for clustering effects due to reader and observation multi-collinearity and compared to the LI-RADS v2018 with McNemar’s test.
Results
224 consecutive patients (180 males; median age, 51 years) were included (Fig. 1). Among them, 196 (88%) patients had chronic HBV infection, and 92 (41%) had established cirrhosis. Of the 742 included liver observations (median size, 13 mm; range, 3–148 mm), 498 (67%) were HCCs, 26 (4%) were non-HCC malignancies, and 218 (29%) were benign lesions.
In the training set, the rLI-RADS diagnostic table was developed with 6 cells, compared to 16 cells for LI-RADS v2018 (Fig. 2C). The %HCC for categories LR-3, LR-4 and LR-5 based on LI-RADS v2018 was 33%, 88%, and 95% in the training set (Fig. 2A) and 27%, 79%, and 94% in the testing set (Fig. 2B), respectively. These measures based on rLI-RADS was 12%, 51%, and 94% in the training set (Fig. 2A) and was 55%, 25%, and 93% in the testing set (Fig. 2B), respectively. The training and testing set weighted kappa value for LI-RADS v2018 categories were 0.807 and 0.798, respectively, and were 0.782 and 0.823 for rLI-RADS, respectively.
In the testing set, rLI-RADS demonstrated a significantly higher sensitivity than LI-RADS v2018 for all observations as a whole (76% vs. 61%, p<0.001), those <10mm (40% vs. 0%, p<0.001), and those between 10-19mm (78% vs. 62%, p<0.001). Likewise, rLI-RADS also yielded a significantly higher accuracy for all observations as a whole (82% vs. 74%, p<0.004), those <10mm (74% vs. 60%, p<0.001), and those between 10-19mm (85% vs. 77%, p<0.001). However, despite with comparable specificities for individual size subgroups (p = 0.125-0.500), the specificity of rLI-RADS was slightly lower than LI-RADS v2018 for all observations as a whole (91% vs. 94%, p=0.008).
Discussions
In a large prospectively-collected cohort, we modified the LI-RADS v2018 diagnostic table while maintaining LI-RADS v2018 feature definitions and overall algorithm design to develop an rLI-RADS system on EOB-MRI. Compared with LI-RADS v2018, rLI-RADS was completely data-driven, remarkably simpler, and demonstrated significantly optimized diagnostic sensitivity and accuracy while maintaining reasonable specificity for HCC.
In this study, we optimized the diagnostic performances by dictating the most appropriate feature combinations of LI-RADS v2018 major features from hard data. Furthermore, system simplicity was also improved driven primarily by the exclusion of lesion size as a criterion from the diagnostic table. Although representing a substantial discordance with LI-RADS v201812 and other diagnostic algorithms13-16, one possible explanation is that the visibility of either washout and/or capsule at imaging may reflect both the underlying physiology and lesion size. Therefore, the associated risk can be captured in the visualization of these findings without requiring size as a separate feature17,18.
Conclusion
Developed based on hard data, rLI-RADS demonstrated superior simplicity, sensitivity and overall accuracy for HCC than v2018 LI-RADS without substantial loss of specificity. Thus, it may be considered the preferred major feature diagnostic system over the original LI-RADS, particularly for early detection purpose.
Acknowledgements
None.References
1. van der Pol CB, Lim CS, Sirlin CB, McGrath TA, Salameh JP, Bashir MR, et al. Accuracy of the Liver Imaging Reporting and Data System in Computed Tomography and Magnetic Resonance Image Analysis of Hepatocellular Carcinoma or Overall Malignancy-A Systematic Review. Gastroenterology. 2019;156(4):976-986. doi: 10.1053/j.gastro.2018.11.020.
2. Elsayes KM, Fowler KJ, Chernyak V, Elmohr MM, Kielar AZ, Hecht E, et al. User and system pitfalls in liver imaging with LI-RADS. J Magn Reson Imaging. 2019;50(6):1673-1686. doi: 10.1002/jmri.26839.
3. Kim DH, Choi SH, Park SH, Kim KW, Byun JH, Kim SY, et al. Meta-analysis of the accuracy of Liver Imaging Reporting and Data System category 4 or 5 for diagnosing hepatocellular carcinoma. Gut. 2019;68(9):1719-1721. doi: 10.1136/gutjnl-2019-318555.
4. Kang JH, Choi SH, Byun JH, Kim DH, Lee SJ, Kim SY, et al. Ancillary features in the Liver Imaging Reporting and Data System: how to improve diagnosis of hepatocellular carcinoma ≤ 3 cm on magnetic resonance imaging. Eur Radiol. 2020;30(5):2881-2889. doi: 10.1007/s00330-019-06645-3.
5. Wei Y, Ye Z, Yuan Y, Huang Z, Wei X, Zhang T, et al. A New Diagnostic Criterion with Gadoxetic Acid-Enhanced MRI May Improve the Diagnostic Performance for Hepatocellular Carcinoma. Liver Cancer. 2020; 9(4):414-425. doi: 10.1159/000505696.
6. Kim DH, Choi SH, Kim SY, Kim MJ, Lee SS, Byun JH. Gadoxetic Acid-enhanced MRI of Hepatocellular Carcinoma: Value of Washout in Transitional and Hepatobiliary Phases. Radiology. 2019;291(3):651-657. doi: 10.1148/radiol.2019182587.
7. Hwang SH, Park S, Han K, Choi JY, Park YN, Park MS. Optimal lexicon of gadoxetic acid-enhanced magnetic resonance imaging for the diagnosis of hepatocellular carcinoma modified from LI-RADS. Abdom Radiol (NY). 2019;44(9):3078-3088. doi: 10.1007/s00261-019-02077-1.
8. Chen J, Kuang S, Zhang Y, Tang W, Xie S, Zhang L, et al. Increasing the sensitivity of LI-RADS v2018 for diagnosis of small (10-19 mm) HCC on extracellular contrast-enhanced MRI. Abdom Radiol (NY). 2020. doi: 10.1007/s00261-020-02790-2. Online ahead of print.
9. Lee S, Kim SS, Bae H, Shin J, Yoon JK, Kim MJ. Application of Liver Imaging Reporting and Data System version 2018 ancillary features to upgrade from LR-4 to LR-5 on gadoxetic acid-enhanced MRI. Eur Radiol. 2020. doi: 10.1007/s00330-020-07146-4. Online ahead of print.
10. Tang A, Bashir MR, Corwin MT, Cruite I, Dietrich CF, Do RKG, et al. Evidence Supporting LI-RADS Major Features for CT- and MR Imaging-based Diagnosis of Hepatocellular Carcinoma: A Systematic Review. Radiology. 2018;286(1):29-48. doi: 10.1148/radiol.2017170554.
11. Cerny M, Bergeron C, Billiard JS, Murphy-Lavallée J, Olivié D, Bérubé J, et al. LI-RADS for MR Imaging Diagnosis of Hepatocellular Carcinoma: Performance of Major and Ancillary Features. Radiology. 2018;288(1):118-128. doi: 10.1148/radiol.2018171678.
12. CT/MRI Liver Imaging Reporting and Data System version 2018. American College of Radiology Web site. https://www.acr.org/Clinical-Resources/Reporting-andData- Systems/LI-RADS/CTMRI-LI-RADS-v2018. Accessed 1 Dec 2018.
13. Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380(15):1450-1462. doi: 10.1056/NEJMra1713263.
14. Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723-750. doi: 10.1002/hep.29913.
15. Department of Medical Administration, National Health and Health Commission of the People's Republic of China. Guidelines for diagnosis and treatment of primary liver cancer in China (2019 edition). Zhonghua Gan Zang Bing Za Zhi. 2020;28(2):112-128. doi: 10.3760/cma.j.issn.1007-3418.2020.02.004.
16. Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11(4):317-370. doi: 10.1007/s12072-017-9799-9.
17. Kitao A, Matsui O, Yoneda N, Kozaka K, Kobayashi S, Koda, et al. Gadoxetic acid-enhanced MR imaging for hepatocellular carcinoma: molecular and genetic background. Eur Radiol. 2020;30(6):3438-3447.
18. Choi JY, Lee JM, Sirlin CB. CT and MR imaging diagnosis and staging of hepatocellular carcinoma: part I. Development, growth, and spread: key pathologic and imaging aspects. Radiology. 2014;272(3):635-654. doi: 10.1148/radiol.14132361.
Figures
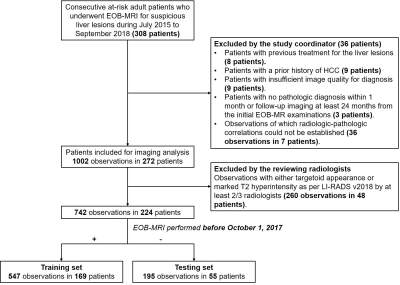
Study flow chart.
EOB-MRI, gadoxetate disodium-enhanced magnetic resonance imaging; HCC, hepatocellular carcinoma; LI-RADS, Liver Imaging Reporting and Data System.
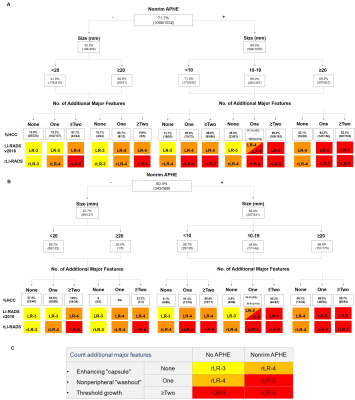
Decision tree illustrating major feature combinations and HCC proportions(%HCC)/counts according to LI-RADS v2018 in the training (A) and testing (B) sets. Data were computed based on sum interpretations of the three readers while adjusted with a generalized estimating equation model. rLI-RADS diagnostic table based on rLR 3-5 category definitions derived from the decision tree (C).
LI-RADS, Liver Imaging Reporting and Data System; rLI-RADS, revised Liver Imaging Reporting and Data System.