2109
A nomogram based on Gd-EOB-DTPA-enhanced MRI to predict early recurrence of hepatocellular carcinoma after curative resection1Department of Radiology, Zhujiang Hospital, Southern Medical University, Guangzhou, China, 2Department of Medical Imaging,Sun Yat-sen University Cancer Center, Guangzhou, China, 3Philips Healthcare, Guangzhou, China
Synopsis
Hepatic resection is the optimal treatment for patients with hepatocellular carcinoma (HCC) in the very early or early stage [Barcelona Clinic Liver Cancer (BCLC)0/A]. However, recurrence within 2 years occurs in 30%–50% of patients; hence, HCC is the major cause of mortality. This study aimed to develop and validate a clinical model to predict the early recurrence of HCC after curative resection. The proposed nomogram provided better discrimination than the BCLC stage and AJCC-TNM (eight edition).
Introduction
Hepatocellular carcinoma (HCC) is the sixth most frequent malignancy and the second most fatal cancer worldwide3. Curative resection is considered as the first-line treatment option for very early or early HCC4. However, tumor recurrence is a primary postoperative complication, which is classified into early recurrence (ER) or late recurrence (LR) based on recurrence within 2 years or beyond6. Establishing and validating an effective model to stratify ER risk in patients with HCC after resection may guide postoperative monitoring, therapeutic interventions, and long-term survival improvement. This study aimed to develop and validate a clinical model to predict the early recurrence of HCC after curative resection.Materials and Methods
331 HCC patients from 2 institutions who underwent curative resection were recruited in this study. The training and test sets comprised 191 and 64 patients from institution 1, and the validation set included 76 patients from institution 2. The clinical or histologic risk factors potentially related to ER included hepatitis B virus DNA quantification (IU/mL), serum AFP level, albumin level (≤35 or >35 g/L), alanine aminotransferase (ALT) (≤50 or>50 U/L), aspartate aminotransferase (AST) (≤40 or >40 U/L),γ-glutamyltransferase (GGT) (≤60 or >60U/L),BCLC stage,AJCC-TNM stage,Edmondson grade, microvascular invasion, and satellite nodules.The threshold values for AFP, ALT, AST, and GGT levels were based on the normal ranges used at institution 1.Two radiologists reviewed all MRI features and evaluated tumor size, tumor number (solitary or multiple), internal arteries [presence of discrete arteries within tumor in the arterial phase (AP)], capsule (a clear, thin enhanced structure surrounding the tumor in the portal venous or delayed phase)5, hypodense halo, and peritumor hypointensity. The enhancement patterns in AP were divided into five types. Type 1 presented a homogenous enhancement pattern with no increase. Type 2 presented an increased homogeneous enhancement pattern. Type 3 presented a heterogeneous and separated enhancement pattern. Type 4 presented a heterogeneous enhancement pattern with irregular ring-like structures. Type 5 presented a heterogeneous nonenhancement6. ER defined as intrahepatic and/or extrahepatic recurrence within 2 years was evaluated by AFP level and typical features of ultrasound and contrast-enhanced CT/MRI that suggested recurrence or confirmed recurrence pathologically during the first 2 years after surgery. Statistical analysis was performed using SPSS 26.0 (IBM, IL, USA) and R software (version 4.0.3; http://www.Rproject.org). Continuous and categorical variables were compared by Mann–Whitney U test and the chi-square test or Fisher’s exact test,respectively. Variables with a P value <0.05 were selected as input variables for multivariate logistic regression analysis to identify independent risk factors of ER in training set. The discriminative performance was quantified by the area under the curve (AUC) of receiver operating characteristic (ROC) curve. The calibration and probabilities of net benefits were quantified by calibration curves and decision curve analysis (DCA), respectively. A two-tailed P value<0.05 was considered statistically significant.Results
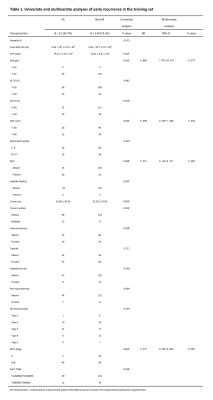
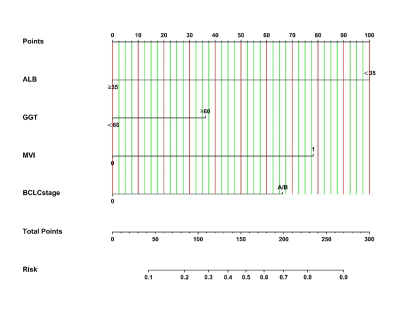
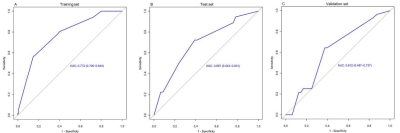
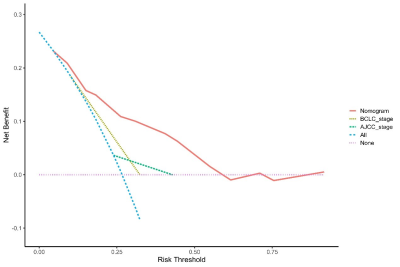
A total of 331 patients were recruited in 3 cohorts from 2 institutions. The ER rate that did not differ in the 3 sets (P = 0.253) was 26.7% (training set, 51 of 191 patients, male:female = 167:24, median age = 53 years), 28.1% (test set, 18 of 64 patients, male:female = 59:5, median age = 54 years), and 36.8% (validation set, 28 of 76 patients, male:female = 70:6, median age = 57). In the training set, the univariate and multivariate logistic regression analyses of 18 clinical-radiologic-pathologic characteristics identified 4 poor independent risk factors related to ER (Table 1). They were ALB>35 g/L) [odds ratio (OR) = 6.880; 95% confidence interval (CI) = 1.370–34.541, P = 0.019), GGT>60 U/L (OR = 0.498; 95% CI = 0.239–1.038, P = 0.063], microvascular invasion (MVI) (OR = 0.221; 95% CI = 0.104–0.471, P = 0.000), and BCLC stage A/B (OR = 0.279; 95% CI = 0.092–0.849, P = 0.025). A nomogram (Fig. 1) as a clinical prognostic model was built. The ROC curves and the AUC were used to evaluate the accuracy of the nomogram (Fig. 2). The AUC of the nomogram in the training set was 0.772 (95% CI: 0.700–0.844), with a sensitivity of 56% and a specificity of 85%. The AUC in the test set was 0.687 (95% CI: 0.543–0.831), with a sensitivity of 72% and a specificity of 61%. The AUC in the validation set was 0.612 (95% CI: 0.487–0.737), with a sensitivity of 64% and a specificity of 63%. The calibration plot for the probability of ER after resection showed an optimal agreement between the prediction by nomogram and actual observation in the three sets (Fig. 3). The DCA for the nomogram, BCLC stage, and AJCC-TNM (eighth edition) is shown in Figure 4. The nomogram was more beneficial than the BCLC stage and AJCC-TNM in predicting .Conclusion
In summary, a nomogram was developed and validated to predict the ER of HCC (≤2 years). The proposed nomogram provided better discrimination than the BCLC stage and AJCC-TNM (eight edition).Acknowledgements
No acknowledgement found.References
1. Bruix J, Sherman M, American Association for the Study of Liver D. Management of hepatocellular carcinoma: an update. Hepatology. Mar 2011;53(3):1020-2. doi:10.1002/hep.24199
2. Chan AWH, Zhong J, Berhane S, et al. Development of pre and post-operative models to predict early recurrence of hepatocellular carcinoma after surgical resection. Journal of Hepatology. 2018;69(6):1284-1293. doi:10.1016/j.jhep.2018.08.027
3. Villanueva A. Hepatocellular Carcinoma. N Engl J Med. Apr 11 2019;380(15):1450-1462. doi:10.1056/NEJMra1713263
4. European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. Jul 2018;69(1):182-236. doi:10.1016/j.jhep.2018.03.019
5. Yang T, Lin C, Zhai J, et al. Surgical resection for advanced hepatocellular carcinoma according to Barcelona Clinic Liver Cancer (BCLC) staging. J Cancer Res Clin Oncol. Jul 2012;138(7):1121-9. doi:10.1007/s00432-012-1188-0
6. Cucchetti A, Piscaglia F, Caturelli E, et al. Comparison of recurrence of hepatocellular carcinoma after resection in patients with cirrhosis to its occurrence in a surveilled cirrhotic population. Ann Surg Oncol. Feb 2009;16(2):413-22. doi:10.1245/s10434-008-0232-4
7. Chan AW, Chan SL, Wong GL, et al. Prognostic Nutritional Index (PNI) Predicts Tumor Recurrence of Very Early/Early Stage Hepatocellular Carcinoma After Surgical Resection. Ann Surg Oncol. Dec 2015;22(13):4138-48. doi:10.1245/s10434-015-4516-1
8. Huang M, Liao B, Xu P, et al. Prediction of Microvascular Invasion in Hepatocellular Carcinoma: Preoperative Gd-EOB-DTPA-Dynamic Enhanced MRI and Histopathological Correlation. Contrast Media Mol Imaging. 2018;2018:9674565. doi:10.1155/2018/9674565
9. Kawamura Y, Ikeda K, Seko Y, et al. Heterogeneous type 4 enhancement of hepatocellular carcinoma on dynamic CT is associated with tumor recurrence after radiofrequency ablation. AJR Am J Roentgenol. Oct 2011;197(4):W665-73. doi:10.2214/AJR.11.6843
10. Wang X, Mao M, He Z, et al. Development and Validation of a Prognostic Nomogram in AFP-negative hepatocellular carcinoma. Int J Biol Sci. 2019;15(1):221-228. doi:10.7150/ijbs.28720
11. Xing H, Zhang WG, Cescon M, et al. Defining and predicting early recurrence after liver resection of hepatocellular carcinoma: a multi-institutional study. HPB (Oxford). May 2020;22(5):677-689. doi:10.1016/j.hpb.2019.09.006
12. Ahn SJ, Kim JH, Park SJ, Kim ST, Han JK. Hepatocellular carcinoma: preoperative gadoxetic acid-enhanced MR imaging can predict early recurrence after curative resection using image features and texture analysis. Abdom Radiol (NY). Feb 2019;44(2):539-548. doi:10.1007/s00261-018-1768-9
Figures