2097
Patient-Specific, In-Vitro Modeling of Aortic Coarctation Using 4D Flow MRI and Particle Image Velocimetry1University of Wisconsin-Madison, Madison, WI, United States, 2Northwestern University, Evanston, IL, United States, 3Lurie Children's Hospital, Chicago, IL, United States
Synopsis
4D flow MRI can be used to assess COA repair hemodynamics in-vivo, albeit, after surgery is performed. This study develops a modeling and validation framework for in-vitro COA hemodynamic assessment. Patient-specific in-vitro models were created representing pre- and post- repair COA aortic geometries. 4D flow was employed to assess model hemodynamics. A PIV protocol was developed to assess the validity of the 4D flow MRI results. PIV velocities qualitatively agreed with 4D flow MRI. In-vitro 4D flow MRI can be used as a predictive tool for COA that can be enhanced with PIV validation.
Introduction
Coarctation of the aorta (COA) is one of the most common congenital heart defects (5-8%), presenting as narrowing of the proximal descending aorta. COA can be discrete or more complex and associated with severe complications such as diffuse arch hypoplasia and mycotic aneurysm. Regardless of clinical presentation, the most common therapy is intervention to restore normal aortic blood flow thus, understanding COA-specific hemodynamics is crucial.1,2 4D flow MRI has shown promise as a tool to assess the hemodynamics of COA in-vivo.3 However, 4D flow MRI data are acquired with a predefined velocity encoding (Venc), set above the maximum expected velocity to avoid velocity aliasing and issues can arise when acquiring data in complex flow regions with both high and low flow velocities, e.g. in patients with COA (high velocity flow jets) and mycotic aneurysm (slow vortex flow velocities) distal to the location of the narrowing.3,4 Pulsatile in-vitro models have proved useful for systematic evaluation of 4D flow performance in complex flow cases by providing a way to produce physiologically realistic geometries and flow conditions that can also be analyzed with higher resolution flow methods, like particle image velocimetry (PIV).5 The goal of this study was to develop a framework for in-vitro modeling of COA hemodynamics using patient-specific aorta COA models, 4D flow MRI and validation through PIV.Methods
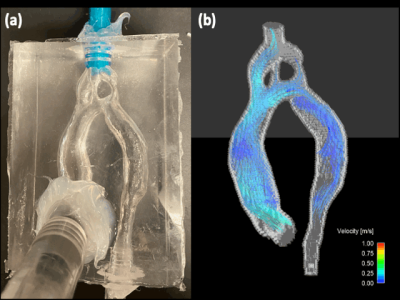
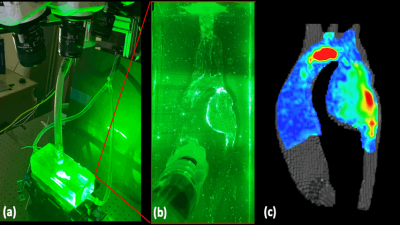
The study included a pediatric patient with aortic COA and a mycotic aneurysm who underwent in-vivo 4D flow MRI pre- and post- COA repair at age 6 and 8 years. 3D contrast-enhanced magnetic resonance angiography (CE-MRA) was used for segmentation of the aortic anatomy and used to construct two in-vitro, patient-specific silicone models (Figure 1). The models were connected to an MRI compatible pulsatile flow circuit and scanned on a 3.0T MRI scanner (Signa Premier, GE, Waukesha, WI) using 4D flow MRI (PC-VIPR technique). Scan parameters were 1.3mm isotropic spatial resolution, 320mm field of view, 150 cm/s velocity encoding and cardiac gaiting. Flow conditions were controlled to match mean aortic flow obtained from in-vivo 4D flow MRI data. PIV was performed on the pre-repair case using a solution, seeded with PMMA fluorescent particles (diameter = 10 mm), of 58% glycerol and 42% water (RI = 1.41) to match the index of refraction of the silicone model, minimizing optical distortions. Data were acquired using a Flowmaster system (LaVision, Göttingen, Germany), consisting of a dual-pulse 527 nm Nd:YLF laser opposite three high-speed cameras (Vision Research, NJ), oriented at -25, 0, and 25 degrees. The light sheet thickness was 5-mm. Data analysis was performed in Ensight (ANSYS Inc, PA).Results
Figures 2 and 3 show the in-vitro 4D flow MRI results pre- and post- repair during the cardiac cycle. Peak pre- and post- repair velocities at the location of the stenosis were recorded as 1.89 and 0.630 m/s, respectively. A high velocity jet was observed for the pre-case upstream of the stenosis and extending to descending aorta. Enhanced flow visualization was achieved in the aneurysm, where vortical flow regions are seen. Post-repair reduction in peak velocity at the location of the coarctation was also observed.Figure 4 shows the PIV experimental setup and velocity visualization of pre-repair velocities at a plane through the center of the aorta. The high velocity jet is shown to accelerate in the aortic arch and extend through the aneurysm with PIV showing initialization of the jet earlier in the aortic arch when compared to 4D Flow MRI, revealing a displacement artifact. Flow visualization at the stenosis location shows unrealistic disturbances potentially induced by imperfections in the model surface. Velocity results within the aneurysm sac provide additional information qualitatively consistent with 4D flow MRI.
Discussion
In this study patient-specific models were fabricated for in-vitro 4D flow MRI experiments to evaluate the blood flow dynamics of COA. The post- repair model showed lower velocities near the stenosis when compared to the pre- case. This agrees with clinical expectation, suggesting 4D flow MRI can be employed to characterize COA hemodynamics, however, future comparisons to actual in-vivo datasets must be made. PIV analysis demonstrated feasibility as a validation framework for COA cases. Velocity maps at the center of the aorta show flow features qualitatively consistent with 4D flow measurements, however, a more robust quantitative analysis is needed. Future direction will concentrate on improving model surface quality and incorporating 3D velocity quantification that can be directly compared to 4D flow MRI results while deriving clinically relevant complex flow metrics such as vorticity and wall shear stress. PIV will also allow for cycle-to-cycle assessment of velocity information, which can account for blood flow dynamics variations not captured with 4D flow MRI.6Conclusion
Use of in vitro models to assess hemodynamics using 4D flow can enhance surgical planning and predictive capabilities in the case of COA. This study established an experimental pipeline to model COA hemodynamics in-vitro utilizing MR compatible COA models of pre- and post- repair aortas and utilized 4D flow MRI and PIV to quantify hemodynamics in-vitro. More robust development of PIV validation is required but shows promise as a tool that can be used to improve COA hemodynamic assessment used to drive clinical treatment planning.Acknowledgements
Grant support by American Heart Association (AHA) 19TPA34850066 and GE Healthcare, which provides research support to the University of Wisconsin-Madison.References
1. Rao P. Coarctation of the aorta. Curr Cardiol Rep. 2005;7(6):425-434.
2. Hope M, Meadows A, Hope T, et al. Clinical evaluation of aortic coarctation with 4D flow MR imaging. J Magn Reson Imaging. 2005;31(3):711-718.
3. Dyverfeldt P, Bissell, M, Barker J, et al. 4D flow cardiovascular magnetic resonance consensus statement. J Cardiovasc Magn Reson. 2015;17(72).
4. Markl M, Fridaychowicz A, Kozerke S, et al. 4D flow MRI. J Magn Reson Imaging. 2012;36(5):1015-1036.
5. Rukowski D, Medero R, Ruesink T, et al. Modeling physiological flow in Fontan models with four-dimensional flow magnetic resonance imaging, particle image velocimetry, and arterial spin labeling. J Biomech Eng. 2019;141(12):121004.
6. Medero R, Ruedinger K, Rutkowski D, et al. In vitro assessment of flow variability in an intercranial aneurysm model using 4D flow MRI and tomographic PIV. Ann Biomech Eng. 2020;48:2484-2493.
Figures