2089
Playing with FIRE: a framework for on-scanner, in-line fully automated 4D-Flow MRI reconstruction, pre-processing and flow visualization1Northwestern, Chicago, IL, United States, 2Cardiovascular MR R&D, Siemens, Chicago, IL, United States
Synopsis
4D-Flow MRI is a valuable technique for quantifying cardiovascular hemodynamics in the aorta; however, it suffers from manual off-line post processing. To address this, we integrated our custom deep learning tools for automatic 4D-Flow processing within the on-scanner reconstruction environment through Siemen’s Framework for Image Reconstruction (FIRE) interface. We retrospectively reconstructed raw data from 10 patients with aortic dilation, valve repair and/or aneurysm as well as one, prospectively recruited, control on scanner. Our deep learning tools ran successfully, and an aortic velocity maximum intensity projection cine was generated and sent to the scanner’s console alongside the reconstructed 4D-flow.
Introduction
4D-Flow MRI has shown to be a valuable technique for evaluating altered cardiovascular hemodynamics in aortic disease including aortic valve abnormalities, dissection, and coarctation.1–3 However, 4D-Flow MRI is currently limited by time intensive, manual off-line post processing, including: eddy current correction, de-noising, velocity anti-aliasing, segmentation of the vessel of interest (e.g. thoracic aorta), and hemodynamic visualization. While there have been significant advancements in automating these tasks through deep learning and artificial intelligence,4,5 the pathway for its full integration within a clinical workflow is unclear. Cloud-based deep learning strategies have been proposed to offload data processing but are limited by upload speeds, concerns regarding data privacy, and limited on-scanner quality assurance regarding the acquisition. To address these limitations, the objective of this study was to fully integrate a 4D-Flow data reconstruction and analysis workflow within the on-scanner image reconstruction pipeline. To achieve this, we utilized the Siemens Framework for Image Reconstruction (FIRE) to augment the standard 4D-Flow MRI reconstruction in order to perform 4D-Flow processing with deep learning derived denoising, eddy current correction, 3D segmentation of the aorta, and visualization of aortic 3D flow dynamics directly on the scanner. We validated this framework in ten patients through computer simulated reconstruction of previously acquired raw data6 and in one healthy control subject.Methods
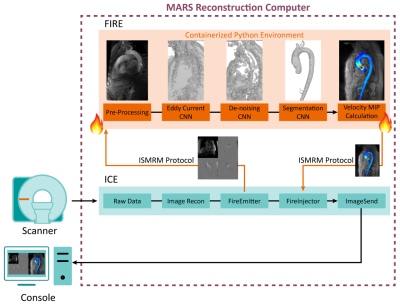
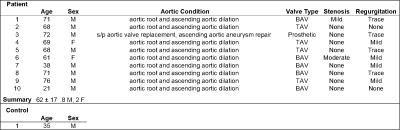
The Siemens FIRE prototype framework provides an open interface between the standard Siemens reconstruction pipeline and a third-party program using the ISMRM raw data7 and image data formats. The pipeline is highly flexible, enabling both custom image reconstruction or processing of semi- to fully-reconstructed data (Figure 1). We deployed our pre-trained deep learning algorithms4,5,8,9 for 4D-Flow image denoising, eddy current correction, and segmentation of the thoracic aorta within a containerized Python 3.6 environment. Scanner reconstructed images, after distortion correction and normalized orientation, were sent to this processing pipeline with FIRE, still within the scanner reconstruction environment. The container performed each processing and deep learning task in a turn-key execution, providing an aortic velocity maximum intensity projection (MIP) cine image for visualization which was delivered to the console alongside the reconstructed 4D-Flow images.To validate this technique, we selected ten 4D-flow data sets from patients with previously collected 4D-Flow MRI raw data with aortic dilation, valve repair, and/or aortic aneurysm (Figure 2) acquired on a 1.5T MAGNETOM Aera (Siemens Healthcare, Erlangen, Germany) with following sequence parameters: GRAPPA R=2, TR=38-41ms,SR=2.3-4.2mm3. Raw data sets were retrospectively reconstructed in the Siemens Integrated Development Environment for Application (IDEA) environment using FIRE and the same 4D-Flow reconstruction code used on the scanner. We additionally validated this technique on-site with one healthy control on a 1.5T MAGNETOM Sola (Siemens Healthcare, Erlangen, Germany) with 4D-Flow prototype acquisition and reconstruction (GRAPPA R=2,TR=41ms ,SR=2.3-4.2mm3, scan time=370s).
Results
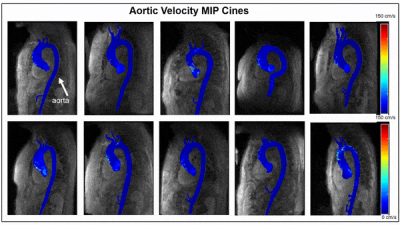
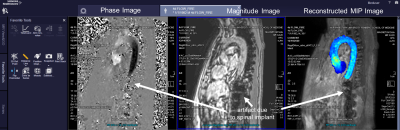
Integration of deep learning post-processing and visualization of 4D-Flow data using the FIRE framework was successful in retrospective reconstruction of all ten patient’s raw data sets and one healthy control on the scanner. For each case, denoising, eddy current correction, and aortic segmentation ran successfully, and an aortic velocity MIP cine was generated and delivered alongside standard reconstructed images. FIRE processing took an average of 64±4 seconds of 211±8 seconds, total, of simulated reconstruction time. Reconstruction time was 185 seconds on scanner for the one control subject. Aortic velocity MIP cines are shown for each patient as a gif (Figure 3). Each MIP image is the maximum intensity projection along the slice direction of the magnitude images, in grayscale for anatomical reference, and a colorized velocity MIP overlay of the segmented aorta. On-scanner 4D-Flow reconstruction with aortic velocity MIP calculation is shown (Figure 4).Discussion
Analysis of 4D-Flow MRI using deep learning algorithms enables automatic post-processing, having the potential to alleviate the time intensive manual processing pipelines that have limited its greater translation. The FIRE framework simplifies the clinical and research workflow through the integration of these algorithms directly to the scanner, requiring no user input. Direct execution in native Python also accelerates translation of algorithmic improvements into the work whenever machine learning models are further refined with additional training data. Close integration between the acquisition, reconstruction, and post-processing may also enable fine tuning of models to account for specific acquisition parameters in the future.Our current implementation provides rapid on-scanner feedback of 4D-Flow acquisitions, visualized using velocity MIP cine images that are more intuitive than standard magnitude and phase image outputs. Flexibility in how custom code is integrated into the reconstruction pipeline with FIRE greatly increases the prototyping capabilities of custom advanced 4D-Flow processing and reconstruction, allowing the rapid translation of existing research tools to the scanner. This study is limited in scope by low number of subjects tested, no quantitative hemodynamic evaluation, or comparison to manual analysis. Future work includes further integration of our analysis tools within the FIRE framework and further quantitative validation of the entire pipeline.
Conclusion
We successfully integrated our deep learning 4D-Flow post-processing algorithms within the Siemens FIRE framework in ten retrospective patients and one prospective subject. Raw data was reconstructed, deep learning algorithms were applied for denoising, eddy current correction, and segmentation, and velocity MIP cines were sent back to the scanner in an automatic pipeline.Acknowledgements
I would like to acknowledge grant support from T32EB025766.References
1. Garcia J, Barker AJ, Markl M. The Role of Imaging of Flow Patterns by 4D Flow MRI in Aortic Stenosis. JACC Cardiovasc Imaging. 2019;12(2):252-266. doi:10.1016/j.jcmg.2018.10.034
2. François CJ, Markl M, Schiebler ML, et al. Four-dimensional, flow-sensitive magnetic resonance imaging of blood flow patterns in thoracic aortic dissections. J Thorac Cardiovasc Surg. 2013;145(5):1359-1366. doi:10.1016/j.jtcvs.2012.07.019
3. Hope MD, Meadows AK, Hope TA, et al. Clinical evaluation of aortic coarctation with 4D flow MR imaging. J Magn Reson Imaging. 2010;31(3):711-718. doi:10.1002/jmri.22083
4. Berhane H, Scott M, Elbaz M, et al. Fully automated 3D aortic segmentation of 4D flow MRI for hemodynamic analysis using deep learning. Magn Reson Med. 2020;84(4):2204-2218. doi:10.1002/mrm.28257
5. Berhane H, Scott M, Robinson J, Rigsby C, Markl M. 3D U-Net for Automated Segmentation of the Thoracic Aorta in 4D-Flow derived 3D PC-MRA. In: ISMRM2019. ; 2019.
6. Pathrose A, Ma L, Berhane H, et al. Highly accelerated aortic 4D flow MRI using compressed sensing: Performance at different acceleration factors in patients with aortic disease. Magn Reson Med. October 2020:mrm.28561. doi:10.1002/mrm.28561
7. Inati SJ, Naegele JD, Zwart NR, et al. ISMRM Raw data format: A proposed standard for MRI raw datasets. Magn Reson Med. 2017;77(1):411-421. doi:10.1002/mrm.26089
8. Berhane H, Scott M, Elbaz M, Markl M. Artificial intelligence-based fullly automated 3D segmentation of the aorta from 4D flow MRI. In: SMRA 2019. ; 2019.
9. Scott M, Berhane H, Robinson JD, Rigsby CK, Markl M. Deep learning for fully automated preprocessing and 3D segmentation of aortic 4D flow MRI. In: Society of Magnetic Resonance Angiography. ; 2019.
Figures