2088
Echo Planar Imaging induced errors in intracardiac 4D MRI flow quantification
Jos J.M. Westenberg1, Hans C van Assen1, Pieter J van den Boogaard1, Jelle J Goeman1, Hicham Saaid2, Jason Voorneveld3, Johan Bosch3, Sasa Kenjeres4, Tom Claessens2, Pankaj Garg5, Marc Kouwenhoven6, and Hildo J Lamb1
1Leiden University Medical Center, Leiden, Netherlands, 2Ghent University, Ghent, Belgium, 3Erasmus Medical Center, Rotterdam, Netherlands, 4University of Technology Delft, Delft, Netherlands, 5Norwich University Hospital, Norwich, United Kingdom, 6Philips Healthcare, Best, Netherlands
1Leiden University Medical Center, Leiden, Netherlands, 2Ghent University, Ghent, Belgium, 3Erasmus Medical Center, Rotterdam, Netherlands, 4University of Technology Delft, Delft, Netherlands, 5Norwich University Hospital, Norwich, United Kingdom, 6Philips Healthcare, Best, Netherlands
Synopsis
Echo Planar Imaging (EPI) is associated with inaccurate velocity quantitation in 4D flow MRI. The systematic errors depend on the orientation of readout and blip phase encoding gradient with respect to the flow direction. This study evaluates EPI-related errors in flow rate and velocity quantitation for in vivo intracardiac 4D flow MRI in a phantom and in ten healthy volunteers. Errors in median flow rate and velocity remain below 10% for normal in vivo intracardiac flow with EPI factor 5. The error is smallest when readout and blip phase encoding gradients are both perpendicular to the main flow.
Introduction
Echo Planar Imaging (EPI)1 using moderate EPI factors was applied for intracardiac blood flow evaluation with 4D flow MRI to enable scanning times below 10 minutes2,3. However, even for moderate EPI factors, systematic quantitative inaccuracies are introduced with EPI4,5. The oscillatory readout gradients create velocity-dependent phase shifts and the unipolar blip phase encoding gradients create motion-induced phase shifts with a modulated point spread function as a result affecting spatial resolution. Dillinger et al.5 recently investigated the limitations of EPI using in silico simulations and in vitro experiments under high-flow conditions. In vivo intracardiac 4D flow was not evaluated. The aim of the current study is to evaluate EPI-related errors in flow rate and velocity quantitation for in vivo intracardiac EPI 4D flow MRI compared to non-EPI gradient echo (GRE) 4D flow MRI.Methods
In vitro experiments on a pulsatile left ventricle (LV) phantom (Figure 1, setup described by Saaid et al.6) and in vivo experiments on ten healthy volunteers (Figure 2, 2/8 female/male, mean age ± standard deviation 37±12 years; informed consent and local Medical Ethical Committee approval was obtained) were performed on 3T MRI (Philips Healthcare). MRI scan parameters are displayed in the table in Figure 3. Whole-heart 4D flow MRI was obtained using standard non-segmented gradient echo (4DGRE) without EPI and with EPI factor 5 (4DEPI). 4DEPI was repeated three times, each with different orientations of readout and blip phase encoding gradient with respect to the main flow direction (Figures 1 and 2): 4DEPI1_LA refers to EPI-acquisition in long-axis orientation with readout gradient aligned parallel to the main flow direction, 4DEPI2_LA refers to EPI-acquisition in long-axis orientation with blip phase encoding gradient aligned parallel to the main flow direction and 4DEPI3_SA refers to EPI-acquisition in short-axis orientation with both readout and blip phase encoding gradients perpendicular to the main flow direction. 4D flow MRI acquisitions were repeated on a phantom filled with static fluid to allow phase offset correction by voxel-wise velocity subtraction7.From each 4D dataset, median LV velocities were obtained from a static cylinder-shaped control volume (in vitro 6,559 measurement voxels, in vivo 1,693±25 measurement voxels) positioned below the mitral valve (MV). Spatial median velocities were compared at peak systole, peak early diastole and over the entire cardiac cycle (i.e., spatio-temporal median). In vivo MV and aortic valve (AoV) net forward volumes (NFVs) were assessed by automated retrospective valve tracking8 using CAAS MR Solutions (Pie Medical Imaging). The difference between MV and AoV NFV determined the consistency in valvular flow assessment. Tracking was performed on cine two-dimensional (2D) left 2-chamber and 4-chamber views and coronal and sagittal views of the LV outflow tract using steady-state free-precession with TE/TR 1.5/2.9 ms, field-of-view 350 mm, 45° flip angle, acquisition resolution 2.0×1.7×8.0 mm3, retrospective gating with 30 phases reconstructed and end-expiration breath holding.
The 4D flow datasets were visually inspected for the presence of artifacts. Relative difference and Pearson correlation (r) between MV and AoV NFV were determined between sequences and compared using paired t-tests. Comparison of median velocities between sequences was done after logarithmic transformation to account for skewness of the distribution, as follows: logarithmic transformation of the median velocity was performed first per cardiac phase, median differences were calculated over the pump or cardiac cycle, respectively, and back transformation resulted in ratios of median velocities between sequences. In vivo, mean ratios and standard deviation between sequences were determined and compared using paired t-tests.
Results
The table in Figure 4 shows median peak velocity magnitudes and interquartile ranges obtained from the control volume inside the LV phantom. Median peak velocity was ≤ 5.5% lower for 4DEPI compared to 4DGRE. Ratios of median velocity comparing 4DEPI with 4DGRE show highest similarity (ratio 0.981) for 4DEPI3_SA with both readout and blip phase encoding gradients perpendicular to the main flow direction and lowest similarity (ratio 0.945) for 4DEPI1_LA with readout gradient aligned parallel with the main flow direction.In vivo, one 4DEPI1_LA dataset was rejected due to presence of unresolved artifacts. The table in Figure 5 shows in vivo results. None of the acquisitions showed statistically significant differences between MV and AoV NFV and all showed strong correlations (r≥0.89, p<0.01). Mean NFV inconsistency was largest (6.4±8.5%) for 4DEPI3_SA with both readout and blip phase encoding gradients perpendicular to the main flow direction. The difference in median LV velocity was largest (9%) for 4DEPI1_LA with readout gradient parallel to the main flow direction.
Conclusion
Flow in the direction of the EPI readout or blip phase encoding gradient results in inaccurate velocity and flow quantitation for EPI-accelerated 4D flow MRI. Errors in median flow rate and median velocity and the inconsistency in MV and AoV NFV all remained below 10% for normal in vivo intracardiac flow with EPI factor 5. The error in flow rate was smallest (<2%) when readout and blip phase encoding gradients are both perpendicular to the main flow direction.Acknowledgements
Contract grant sponsor: Dutch Heart Foundation; Contract grant number: CVON2017-08-RADAR (for second author)References
1. DeLaPaz RL. Echo-planar Imaging. Radiographics 1994; 14:1045-58.2. Kozerke S, Hasenkam JM, Pedersen EM, Boesiger P. Visualization of flow patterns distal to aortic valve prostheses in humans using a fast approach for cine 3D velocity mapping. J Magn Reson Imaging 2001; 13(5):690-698.
3. Westenberg JJ, Roes SD, Ajmone Marsan N, et al. Mitral valve and tricuspid valve blood flow: accurate quantification with 3D velocity-encoded MR imaging with retrospective valve tracking. Radiology. 2008; 249(3):792-800.
4. Duerk JL, Simonetti OP. Theoretical aspects of motion sensitivity and compensation in echo-planar imaging. J Magn Reson Imaging. 1991; 1:643–650.
5. Dillinger H, Walheim J, Kozerke S. On the limitations of echo planar 4D flow MRI. Magn Reson Med. 2020; 84(4):1806-1816.
6. Saaid H, Voorneveld J, Schinkel C, et al. Tomographic PIV in a model of the left ventricle: 3D flow past biological and mechanical heart valves. J Biomech. 2019; 90:40-49.
7. Hofman MBM, Rodenburg MJA, Markenroth Bloch K, et al. In-vivo validation of interpolation-based phase offset correction in cardiovascular magnetic resonance flow quantification: a multi-vendor, multi-center study. J Cardiovasc Magn Reson. 2019; 21(1):30.
8. Kamphuis VP, Roest AAW, Ajmone Marsan N, et al. Automated cardiac valve tracking for flow quantification with four-dimensional flow MRI. Radiology 2019; 290(1):70-78.
Figures
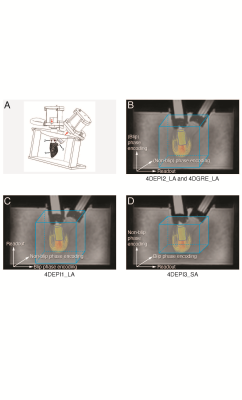
In A,
1 indicates the LV phantom, 2 and 3 indicate bioprosthetic mitral and aortic
valve. In B-D, gradient orientations for 4DEPI and 4DGRE are shown. 4DEPI1_LA: long-axis
oriented EPI with readout gradient parallel to the main flow, 4DEPI2_LA: long-axis
oriented EPI with blip phase encoding gradient parallel to the main flow, 4DEPI3_SA:
short-axis oriented EPI with both readout and blip phase encoding gradients perpendicular
to the main flow. A cylinder-shaped control volume is positioned below the
mitral valve.
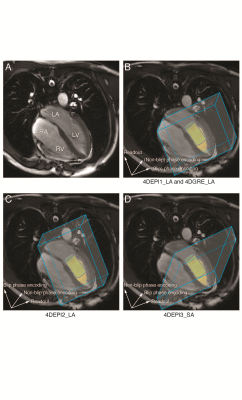
In
A, the 4-chamber is shown (LV: left ventricle, LA: left atrium, RV: right
ventricle, RA: right atrium). In B-D, gradient orientations for 4DEPI and 4DGRE
are shown, similar as for the LV phantom. 4DEPI1_LA: long-axis oriented EPI with
readout gradient parallel to the main flow, 4DEPI2_LA: long-axis oriented EPI with
blip phase encoding gradient parallel to the main flow, 4DEPI3_SA: short-axis
oriented EPI with both readout and blip phase encoding gradients perpendicular
to the main flow. A cylinder-shaped control volume is positioned below the
mitral valve.
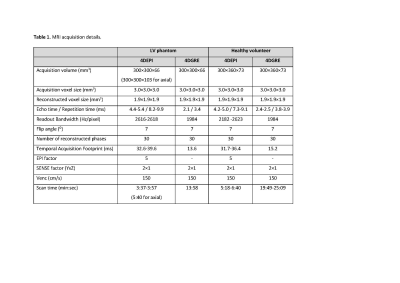
MRI
scan parameters and acquisition details.
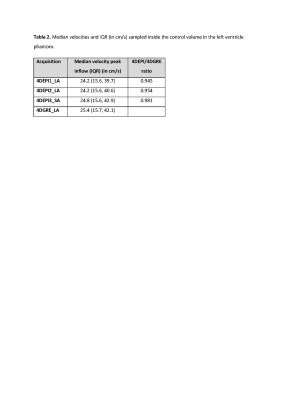
Median
velocities and IQR (in cm/s) sampled inside the control volume in the left
ventricle phantom. 4DEPI1_LA: long-axis oriented EPI with readout gradient parallel to the main flow, 4DEPI2_LA: long-axis oriented EPI with blip phase encoding gradient parallel to the main flow, 4DEPI3_SA: short-axis oriented EPI with both readout and blip phase encoding gradients perpendicular to the main flow. Abbreviations: EPI: echo planar imaging, GRE: gradient echo, LA:
long-axis, SA: short-axis.
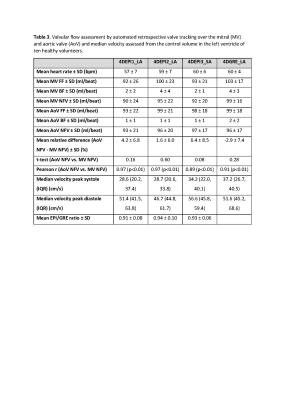
Mitral (MV)
and aortic valve (AoV) flows and median velocities sampled inside control volume in left ventricle of ten healthy volunteers. 4DEPI1_LA: long-axis oriented EPI with readout gradient parallel to main flow, 4DEPI2_LA: long-axis oriented EPI with blip phase encoding gradient parallel to main flow, 4DEPI3_SA: short-axis oriented EPI with both readout and blip phase encoding gradients perpendicular to main flow. Abbreviations: EPI: echo planar imaging, GRE: gradient echo, LA: long-axis, SA: short-axis, FF: forward
flow, BF: backward flow, NFV: net forward volume.