2078
Computationally Enhanced 4D Flow MRI for the Assessment of Pre- and Post-Coarctation Repair Aorta Flow Dynamics1University of Wisconsin-Madison, Madison, WI, United States, 2Northwestern University, Chicago, IL, United States, 3Ann & Robert H. Lurie Children's Hospital of Chicago, Chicago, IL, United States
Synopsis
CMR is used for diagnosis of COA and post-intervention assessment. Current limitations of 4D flow MRI include insufficient spatio-temporal resolution, inability to image low velocity regions, and distortion of image due to stent. The presented MRI-based computational enhancement method addresses stated limitations. Patient specific flow velocities defined the boundary conditions for numerical simulations. CFD simulations with AMR were run on segmented aorta geometries. Comparison between in-vivo and CFD results show good agreement, and residual complex flows post-repair compared to healthy control. Computational enhancement of 4D flow MRI can be used as a predictive tool in future clinical settings.
Introduction
Coarctation of the aorta (COA) is a congenital heart disease characterized by a narrowing in the aortic arch or proximal descending aorta [1]. COA results in altered aortic flow dynamics (elevated pressure gradient, deranged flow) and subsequent complications such as post-stenotic aneurysms or insufficient blood supply to the periphery. Interventional treatment such as surgery, stent placement, and balloon angioplasty are often performed to restore normal aortic hemodynamics [2]. Diagnostic imaging, such as echocardiogram and cardiovascular MR (CMR), for aortic hemodynamics is important for intervention planning and assessment of treatment success. Despite advances in CMR such as 4D flow MRI for in-vivo analysis of cardiovascular flow dynamics, current techniques have limitations in phase offset errors, insufficient spatio-temporal resolution, and distortion of images due to stent (metal artifact), and thus unreliable quantification of flow metrics. Methods have been developed to better predict hemodynamic outcomes using patient specific CMR data supplemented by computational models of cardiovascular fluid dynamics. The purpose of this study was to evaluate the feasibility of patient specific MRI-based computational models using a novel method called adaptive mesh refinement (AMR) for improved quantitative flow dynamics analysis in patients with different types of COA repair.Methods
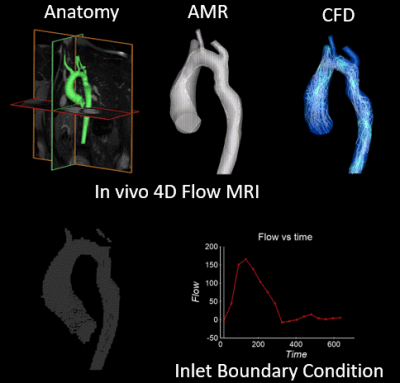
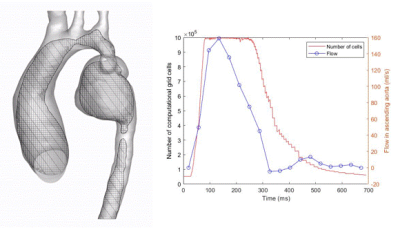
In-vivo 4D flow MRI was performed on three pediatric subjects following an IRB-approved protocol. Patient 1, a pediatric patient with a mycotic aneurysm, was scanned before and after surgical repair with interposition graft (at ages 6 and 8). Patient 2 presented with COA and severe aortic kinking in the proximal descending aorta and underwent 4D flow MRI before and after repair by stent placement (at ages 14 and 17). The third subject was a healthy control (age 10). For all subjects, 3D segmentation of the aorta was performed (MIMICs) based on contrast enhanced MRA (CE-MRA) or CT scans, and a total of 5 models of the aorta (2 patients pre- and post COA repair, 1 healthy control) were generated for numerical simulations. Flow velocities derived from in-vivo 4D flow MRI studies (EnSight, CEI) were used to define patient specific boundary conditions. Computational fluid dynamics (CFD) simulations were conducted on Converge software using AMR. AMR refines the local computational grid size based on previously calculated discretization error [3]. Implementing AMR reduces the computational resources required for the CFD simulations while maintaining high accuracy in the numerical results. Figure 1 shows the schematic of this computational method enhancing 4D flow MRI for cardiovascular flow dynamics analysis for patient 2 before stent placement. Figure 2 shows the implementation of AMR on patient 1 pre-surgery. Velocities from CFD simulations were compared with 4D flow MRI derived in-vivo velocities.Results
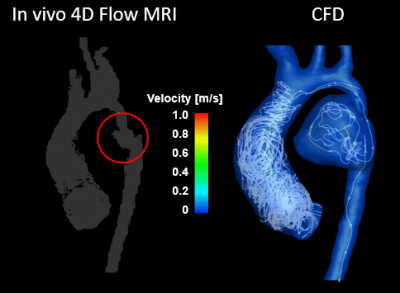
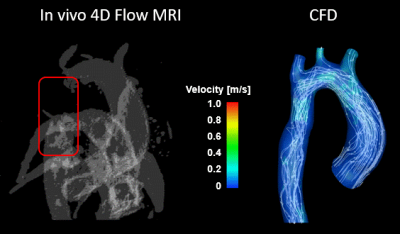
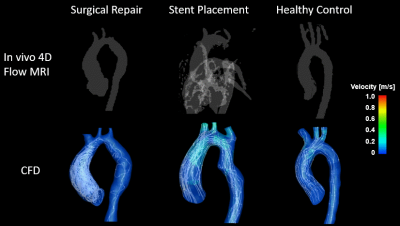
A total of five CFD simulations were successfully executed using patient specific anatomical geometries and flow conditions. For patient 1, the differences in mean velocities (at systole and diastole) between CFD and 4D flow studies were 5.4% and 13.3% in the ascending and descending regions, respectively. For patient 2, differences (at systole and diastole) were 0.3% and 1.8% in the ascending and descending regions, respectively. The locations of vortical flow from CFD simulations matched those from 4D flow MRI data. Figure 3 shows in-vivo 4D flow MRI and CFD results for patient 1 before intervention. CFD simulations revealed flow inside the aneurysm previously not captured by 4D flow MRI due low flow velocities. Figure 4 shows in-vivo 4D flow MRI and CFD results for patient 2 after stent placement. 4D flow MRI results were clearly distorted due to metal artifact of the stent. Notably, numerical simulations could recover flow dynamics inside and distal to the tendered region that could not be resolved by in-vivo 4D flow MRI. The temporal resolution of enhanced CFD method was higher with a timestep size of 10 ms, compared to 38.5 ms for 4D flow MRI. The computationally enhanced technique showed better spatial resolution as well, resulting in visualization of flow in the aneurysm (Figure 3) and stent (Figure 4). The post-intervention cases were compared with the in-vivo 4D flow MRI and CFD results of the healthy control (Figure 5). Qualitative analysis of in-vivo and CFD flow dynamics show that post repair cases had residual complexities in the hemodynamic flow with larger vortices in the ascending region.Discussion
Computationally enhanced 4D flow MRI with AMR improved the spatio-temporal resolution. AMR based CFD successfully restored flow metrics in regions of low velocity in the patient 1 aneurysm, likely due to low aneurysmal velocities relative to the VENC. This method also predicted cardiovascular flow inside and surrounding the stent in patient 2. Further, CFD provided flow information with good qualitative agreement with in-vivo results. In addition, mean velocities at ascending and descending aortic regions (at systole and diastole) using this method showed agreement with in-vivo data to within 15% difference.Conclusion
The goal of COA repair is to restore normal hemodynamics. Despite improvements, CFD shows residual complex flow regions post-repair when compared to the healthy case. The novel AMR-augmented 4D flow MRI is feasible to derive quantitative flow metrics. This treatment method can be used as a predictive tool for treatment planning and post-intervention assessment in future patient specific clinical applications.Acknowledgements
Grant support by American Heart Association (AHA) 19TPA34850066
GE Healthcare, which provides research support to the University of Wisconsin
References
[1] P. S. Rao, “Coarctation of the Aorta,” Curr. Cardiol. Rep., vol. 7, pp. 425–434, 2005.
[2] A. Saxena, “Proceedings of the Fourth Scientific Meeting of the World Society for Pediatric and Congenital Heart Surgery Recurrent Coarctation: Interventional Techniques and Results,” doi: 10.1177/2150135114566099.
[3] O. Sahni, K. E. Jansen, C. A. Taylor, and M. S. Shephard, “Automated adaptive cardiovascular flow simulations,” Eng. Comput., vol. 25, no. 1, pp. 25–36, 2009, doi: 10.1007/s00366-008-0110-5.
Figures