2073
Anatomical location related hemodynamic changes assessed by 4D flow MRI in the intracranial arteries of young secondary hypertensive patients1Department of Magnetic Resonance, Lanzhou University Second Hospital, Lanzhou, China, 2Philips Healthcare, Xi'an, China
Synopsis
The multi-parameter analysis of 4D flow MRI can identify the changes of hemodynamic parameters in different anatomical location of intracranial arteries. This study evaluating the hemodynamic changes (volume, velocity, wall shear stress) in the intracranial arteries of young secondary hypertensive patients among different anatomical locations (ICA-C3, ICA-C7, proximal MCA, proximal PCA, middle BA) using 4D flow MRI. The proximal PCA had significantly lower volume, velocity and wall shear stress than the values determined for other locations. The wall shear stress at the anatomical location of ICA-C3 was decreased in young secondary hypertensive patients.
Introduction
Cardiovascular disease is a serious disease that can cause more than 18 million deaths each year. Hypertension is a recognized risk factor for cardiovascular disease. Recent epidemiological studies have shown that the incidence of young people is gradually increasing. 4D flow MRI can provide a series of hemodynamic parameters: wall shear Stress (WSS), pressure difference, vortex flow energy, velocity and volume, etc. At present, the research of 4D flow MRI technology in intracranial vascular disease mainly focuses on intracranial aneurysms and arteriovenous malformations (AVM). We used 4D flow MRI to evaluate hemodynamic changes (volume, velocity, WSS) in the intracranial arteries of young secondary hypertensive patients among different anatomical locations.Methods
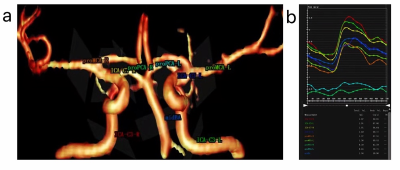
Twenty-three young secondary hypertensive patients aged 20-45 years and twenty-three age-matched healthy volunteers were enrolled in this study. 4D flow MRI examinations were performed for each subject. All subjects were scanned using a 3.0 T MR scanner (Ingenia CX, Philips Healthcare, the Netherlands) with a 32 channel head coil. The 4D flow MRI was acquired using a volumetric and a time-resolved PC method. The scanning parameters were as follows: FOV, 20×20×10cm; reconstruction resolution, acquired isotropic spatial resolution=1mm3; velocity encoding, 80cm/s; TR/TE, 5.6/2.9ms; flip angle, 8°;20 phases. The total scan time of the 4D flow took approximately 12−15 minutes depending on the heart rate of each subject. The 4D flow datasets were imported into the CVI-42 platform (Version 5.6.6, Circle Cardiovascular Imaging, Canada) for further analysis. As displayed in Fig.1a and b, we placed nine planes in the intracranial arteries (ICA-C3-R, ICA-C3-L, ICA-C7-R, ICA-C7-L, proMCA-R, proMCA-L, proPCA-R, proPCA-L and mid-BA) to evaluate the volume, velocity, WSSmax and WSSmean. Statistical analysis was performed using SPSS (version 25.0, Chicago, IL) software. Independent two sample t-tests were used to detect hemodynamic changes among young secondary hypertensive patients and healthy adults. The influence of different anatomical locations on hemodynamic parameters was using multivariate variance analysis. Paired t-tests were used for left/right vessels in different anatomical locations. P<0.05 was considered statistically significant.Results
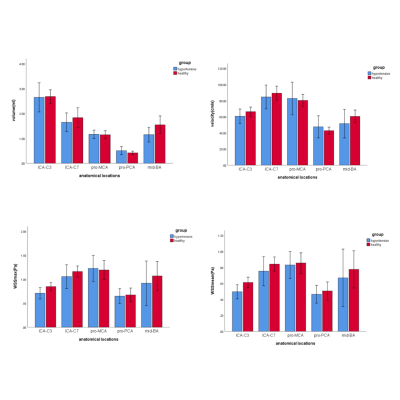
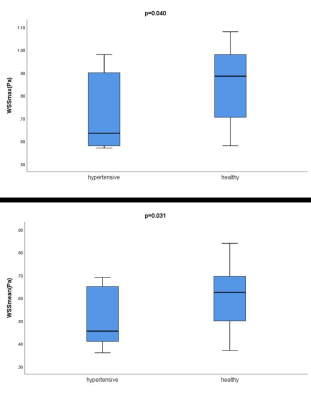
There were no differences between the left and right intracranial arteries for any of the hemodynamic parameters (all p values>0.05). Hemodynamic changes in different locations of the intracranial arteries are displayed in Fig.2. The proximal PCA had significantly lower volume, velocity and WSS than the values determined for other locations (p<0.05). As displayed in Fig.3, compared with healthy adults, young secondary hypertensive patients have lower WSSmax and WSSmean at the anatomical location of ICA-C3 (p<0.05).Discussion
4D flow MRI is an emerging tool for the evaluation of cardiovascular hemodynamics with full volumetric coverage. It measures the flow velocity directly in vivo and has been mostly used in the cardiovascular system in clinical practice[1]. Our results showed no differences in the bilateral intracranial arteries, indicating that the origin of the blood vessels may not cause the difference in hemodynamics. Hypertensive vascular disease is mainly characterized by thickening and poor elasticity of blood vessel walls, which can lead to atherosclerosis, aneurysm and aortic dissection. The vessel diameter can increase with hypertensive due to its decreased elasticity[2], which may contribute to the decreased velocity. This state of hemodynamics led to the formation of atherosclerosis, aneurysm and aortic dissection. It is now widely accepted that low WSS is an essential factor in promoting plaque formation[3]. To some extent, our study in line with this theory. The WSSmax and WSSmean demonstrated a reduction in young secondary hypertensive patients. The low WSS increased the uptake of oxidized low-density lipoprotein[4], causing increasing lipid components to form in plaques. Moreover, the low WSS altered the vascular endothelium flow patterns at the molecular and cellular levels. All these reactions promote the development of atherosclerosis[5].Conclusion
As an initial and exploratory study, this work showed that young secondary hypertensive patients have lower WSSmax and WSSmean at the anatomical location of ICA-C3, compared with healthy adults. This may imply that low WSS is more likely to cause atherosclerosis, aneurysm and aortic dissection. This indicates that hypertension and anatomical location impacted hemodynamic.Acknowledgements
Health Industry Scientific Research Program Project of Gansu Province (Project Number: GSWSKY-2019-09)References
[1] Holmgren M, Wåhlin A, Dunås T, et al. Assessment of cerebral blood flow pulsatility and cerebral arterial compliance with 4d flow mri[J]. Journal of Magnetic Resonance Imaging, 2020, 51(5): 1516-1525.
[2] Birnefeld J, Wåhlin A, Eklund A, et al. Cerebral arterial pulsatility is associated with features of small vessel disease in patients with acute stroke and TIA: a 4D flow MRI study[J]. Journal of Neurology, 2020, 267(3): 721-730.
[3] Wolak A, Gransar H, Thomson L E J, et al. Aortic size assessment by noncontrast cardiac computed tomography: normal limits by age, gender, and body surface area[J]. JACC: Cardiovascular Imaging, 2008, 1(2): 200-209.
[4] Miller K B, Howery A J, Rivera-Rivera L A, et al. Age-related reductions in cerebrovascular reactivity using 4D flow MRI[J]. Frontiers in aging neuroscience, 2019, 11: 281.
[5] Zhang G, Wang Z, Zhang S, et al. Age and anatomical location related hemodynamic changes assessed by 4D flow MRI in the carotid arteries of healthy adults[J]. European Journal of Radiology, 2020: 109035.
Figures