2067
Pulsatility attenuation along the carotid siphon in pseudoxanthoma elasticum1Radiology, UMC Utrecht, Utrecht, Netherlands, 2Neurology, UMC Utrecht, Utrecht, Netherlands, 3Vascular Medicine, UMC Utrecht, Utrecht, Netherlands
Synopsis
Arterial internal elastic lamina calcifications in pseudoxanthoma elasticum (PXE) may reduce the normal attenuation by the carotid siphon. This could contribute to increased intracranial pulsatility seen in PXE patients. We compared velocity pulsatility and distensibility along the internal carotid artery and in the middle cerebral artery between 50 PXE patients and 40 age and sex-matched controls, using 2D phase-contrast velocity mapping on 3T MRI. Distensibility was lower and pulsatility higher in patients. However, pulsatility attenuation over the siphon was similar between patients and controls. Siphon dysfunction does, therefore, not explain the increased intracranial arterial pulsatility in PXE.
Introduction
The distal internal carotid artery (ICA), called the carotid siphon, is a tortuous segment which is located in the cavernous sinus and attenuates arterial pulsatility (1,2). Arterial calcification in the siphon and related stiffening might lead to reduced pulse pressure attenuation which results in increased intracranial arterial pulsatility. Patients with the rare, hereditary disorder pseudoxanthoma elasticum (PXE) develop severe calcification in the skin, eyes and in the internal elastic lamina of arteries. PXE patients are at increased risk for stroke and small vessel disease (3,4). The presence of non-atherosclerotic intracranial internal carotid artery calcification (iICAC) as seen in PXE is also common in the general population where it co-exists with atherosclerotic calcifications (5,6). Both iICAC and increased blood flow pulsatility are known risk factors for stroke and cerebral small vessel disease (SVD) in the general population. Calcification probably contributes to increased velocity pulsatility by stiffening of the arterial wall at the site of calcification, which hampers the attenuation of the heartbeat induced pulse pressure upon its traverse to the microvascular bed. The observed predominant location of iICAC in the carotid siphon, the association of calcifications with stroke risk, and the potential role of the siphon as pulsatility attenuator (1,2) raise the question whether pulsatility damping by the siphon is different in PXE patients compared to matched controls. To investigate whether pulsatility damping by the carotid siphon is different in PXE patients, we compared the velocity pulsatility and distensibility along the trajectory of the ICA and in the middle cerebral artery (MCA) between PXE patients and controls.Methods
Fifty PXE patients had a confirmed clinical diagnosis based on the criteria of Plomp et al. (2010) (7). Forty age and sex-matched controls were recruited from the families and acquaintances of PXE patients excluding first or second degree relatives. The study was approved by the institutional review board of the UMCU. All participants gave written informed consent. All participants were scanned on 3T MRI with a 32-channel head coil (Philips, Best, The Netherlands). 2-dimensional phase-contrast (2D PC) MRI acquisitions were used to acquire time-resolved measurements of blood velocities and volumetric flow rates proximal at the beginning of the C4 segment and distal to the carotid siphon (C6 segment) (8), and in the MCA. A 2D radiofrequency-spoiled gradient-echo sequence with retrospective cardiac gating and unidirectional through-plane velocity encoding was used with the following imaging parameters: 250x250mm field of view, acquired spatial resolution 0.5x0.5x3mm3 and an acquired temporal resolution of 64 ms. Velocity encoding (VENC) sensitivity was 100 cm/s for C4, 150 cm/s for C6 and 100 cm/s for MCA. The flow acquisitions resulted in a series of 2D images, representing the blood-flow velocity in consecutive timeframes of the cardiac cycle. The minimum, maximum and mean blood flow velocities (Vmin, Vmax and Vmean) in cm/s, were used to calculate the PI ((Vmax-Vmin)/Vmean) from each velocity curve (9). Arterial distensibility, (((Amax-Amin)/Amean)/ΔP)*100 was calculated from each area curve, where D indicates the diameter of the ROI and ΔP is systolic pressure – diastolic pressure (10). Blood pressure was measured on the day of study participation (lowest measurement out of 4). Descriptive data was presented as mean ± SD for normally distributed variables, median (IQR) for non-normally distributed variables and n (%) for categorical variables. Differences between the PXE and control group were tested with the students t-test, Mann-Whitney U test or χ2 test when appropriate. Data analysis was performed in RStudio v1.1.456, a p-value <0.05 was regarded statistically significant.Results / Discussion
Fifty PXE patients (57±12 years old, 49% male) and 40 controls (58±11 years old, 50% male) were enrolled between June 2017 and October 2019 (Table 1). One PXE patient with an ophthalmic artery aneurysm was excluded because this might affect flow and pulsatility attenuation in the carotid siphon. PI decreased from C4 to C6 and from C6 to the MCA in both PXE patients and controls (Table 1). PXE patients had higher pulsatility index than controls in all measured segments. Distensibility was lower in PXE than in controls in C6 (p<0.01) and the MCA (p<0.01), but not in the C4 segment, where the ICA passes through the skull base (Table 1, Figure 1). In PXE patients, pulsatility decreased slightly less between C4 and C6 than in controls (p=0.03), but the effective attenuation between C4 and the MCA was very similar (p=0.43, Table 1, Figure 2). The current finding is different from our observations in patients with SVD without PXE, where pulsatility increased over the carotid siphon compared to controls [unpublished data]. Although the number of patients in both studies were rather small, these findings suggest that the arterial phenotype of PXE is not necessarily a representative model for the hemodynamics in SVD patients. This might be due to the fact that SVD patients typically have combined intimal disease and medial arterial calcification, whereas PXE patients have relatively isolated medial arterial calcification.Conclusion
In conclusion, despite lower distensibility, there is no difference in pulsatility attenuation between PXE patients and controls. This suggests that other disease mechanisms, e.g. extracranial calcification and stiffness, rather than siphon dysfunction, mainly contribute to increased intracranial arterial pulsatility in PXE.Acknowledgements
We thank the study participants and magnetic resonance technicians for their support and participation.References
1. van Tuijl RJ, Ruigrok YM, Velthuis BK, van der Schaaf IC, Rinkel GJE, Zwanenburg JJM. Velocity Pulsatility and Arterial Distensibility Along the Internal Carotid Artery. J Am Heart Assoc. 2020;9(16):e016883.
2. Schubert T, Santini F, Stalder AF, et al. Dampening of blood-flow pulsatility along the carotid siphon: Does form follow function? Am J Neuroradiol. 2011;32(6):1107-1112.
3. Vos A, Kranenburg G, de Jong PA, et al. The amount of calcifications in pseudoxanthoma elasticum patients is underestimated in computed tomographic imaging; a post-mortem correlation of histological and computed tomographic findings in two cases. Insights Imaging. 2018;9(4):493-498.
4. Kauw F, Kranenburg G, Kappelle LJ, et al. Cerebral disease in a nationwide Dutch pseudoxanthoma elasticum cohort with a systematic review of the literature. J Neurol Sci. 2017;373:167-172.
5. Bartstra JW, van den Beukel TC, Van Hecke W, et al. Intracranial Arterial Calcification: Prevalence, Risk Factors, and Consequences: JACC Review Topic of the Week. J Am Coll Cardiol. 2020;76(13):1595-1604.
6. Vos A, Van Hecke W, Spliet WGM, et al. Predominance of nonatherosclerotic internal elastic lamina calcification in the intracranial internal carotid artery. Stroke. 2016;47(1):221-223.
7. Plomp AS, Toonstra J, Bergen AAB, Van Dijk MR, De Jong PTVM. Proposal for updating the pseudoxanthoma elasticum classification system and a review of the clinical findings. Am J Med Genet Part A. 2010;152(4):1049-1058.
8. Bouthillier A, Van Loveren HR, Keller JT. Segments of the internal carotid artery: A new classification. Neurosurgery. 1996;38(3):425-433.
9. Gosling RG, Dunbar G, King DH, et al. The Quantitative Analysis of Occlusive Peripheral Arterial Disease By a Non-Intrusive Ultrasonic Technique. Angiology. 1971;22(1):52-55.
10. O’Rourke MF, Staessen JA, Vlachopoulos C, Duprez D, Plante GE. Clinical applications of arterial stiffness; definitions and reference values. Am J Hypertens. 2002;15(5):426-444.
Figures
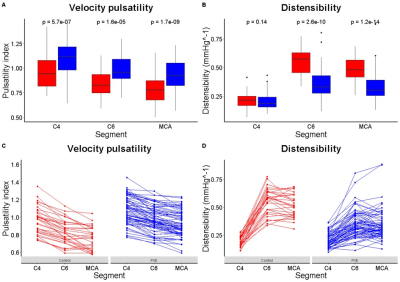
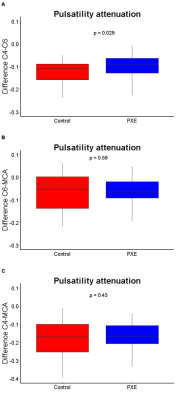
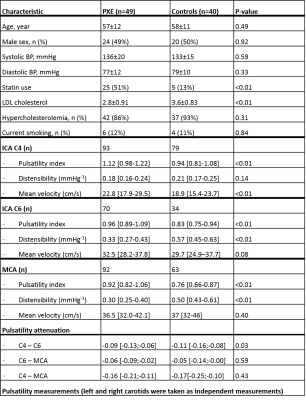
Table 1: Baseline characteristics and pulsatility pseudoxanthoma elasticum (PXE) patients and controls.
Data = mean ± SD, median [Q1-Q3] or n (%). students t-test, Mann-Whitney U test or χ2 test when appropriate. P-value<0.05 was regarded statistically significant. BP = blood pressure, ICA = internal carotid artery, LDL = low density lipoprotein, MCA = middle cerebral artery.