2058
Microstructural Gray Matter Abnormalities in Progressive Supranuclear Palsy and Corticobasal Syndrome: Evaluation by Free-water Imaging1Department of Radiology, Juntendo University Graduate School of Medicine, Tokyo, Japan, 2Department of Radiological Sciences, Tokyo Metropolitan University, Tokyo, Japan, 3Department of Radiology, Toho University Omori Medical Center, Tokyo, Japan
Synopsis
Corticobasal syndrome (CBS) and progressive supranuclear palsy (PSP) are neurodegenerative disorders characterized by the deposition of the abnormal 4 microtubule-binding domain (4R) tau protein in specific brain regions. Voxel-based morphometry (VBM) has been widely used in CBS and PSD; however, the detection of pathological changes of these diseases is challenging in the early stages, including with VBM. In this study, we used free-water imaging (FWI) to evaluate gray matter (GM) microstructural changes in CBS and PSP. We determined that FWI could detect GM abnormalities, which likely reflected tau-related pathology in CBS and PSP patients more sensitively than VBM.
Introduction
Corticobasal syndrome (CBS) and progressive supranuclear palsy (PSP) are neurodegenerative disorders characterized by the deposition of abnormal 4 microtubule-binding domain (4R) tau protein in specific brain regions. 1 Previous neuroimaging studies focused on measuring the atrophy of local gray matter (GM) to detect pathological changes of CBS and PSP in the GM. 2, 3 However, the evaluation of microstructural changes of GM has recently become possible using diffusion magnetic resonance imaging (MRI) with higher sensitivity than GM atrophy. 4 Diffusion tensor imaging (DTI) is commonly used to investigate brain microstructural abnormalities. However, the DTI model assumes a single-tissue compartment per voxel, which generates biased DTI metrics in voxels consisting of a mixture of tissue and free water (FW). This problem becomes apparent in the assessment of the cortex because of the close proximity of the cortex and cerebrospinal fluid (CSF; i.e. FW). In this study, we propose the use of a novel diffusion MRI technique called free-water imaging (FWI) 5 that enables elimination of the contribution of FW to the DTI assessment to evaluate GM microstructural changes in CBS and PSP.Methods
Study participantsThis study included 16 CBS and 16 PSP patients from the Four Repeat Tauopathy Neuroimaging Initiative (4RTNI*) database and 17 healthy controls from the Neuroimaging Initiative for Frontotemporal Lobar Degeneration (FTLDNI*). Figure 1 summarizes the demographic characteristics.
MR imaging
We performed diffusion MRI using a single-shot EPI sequence with the following scan parameters: TR/TE = 9200/82 ms, matrix = 128 × 128, number of slices = 44, resolution = 2.7 mm3. Four b0 images and b = 1000 s/mm2 images with 41-gradient directions were acquired using parallel imaging. DTI (FA, MD, AD, and RD) and FWI (FAt, MDt, ADt, RDt, and FW) were subsequently obtained.
GBSS
GM-based spatial statistics (GBSS)6 were used to regionally map significant group differences in each of the DTI and FWI metrics. We used GBSS to exclude the effects of the partial volume effect from the white matter and CSF that might lead to errors while measuring the dMRI parameters in the cerebral cortex.
VBM
GM volume was evaluated with VBM using Statistical Parametric Mapping 12 (SPM12) software. The diffeomorphic anatomical registration using exponentiated lie algebra (DARTEL) was used to spatially normalize the GM maps to the Montreal Neurological Institute (MNI) space.
ROI
Region-of-interest (ROI) analysis was performed using the Desikan–Killiany cortical and subcortical atlas.7 DTI and FWI measures were subsequently measured in six regions (precentral gyrus, postcentral gyrus, superior frontal gyrus, inferior temporal gyrus, thalamus, and putamen) described in the previous literature as vulnerable to tau pathology8 (Figure 2).
Statistical analysis
For the GBSS analysis, voxel-wise statistics of the diffusion metrics for group differences were tested in the general linear model (GLM) framework with analysis of variance, using the Randomize tool with nonparametric permutation testing with age and gender as covariates. For the VBM analysis, GM volume was compared between groups using GLM analysis of covariance, with age, gender, and total brain volume included as nuisance covariates. A familywise error–corrected p value <0.05 was considered significant. For the ROI analysis, mean diffusion metrics in the GM were compared among groups using the Kruskal–Wallis test. To detect significant main effects on any area, pairwise comparisons were then performed with significant Bonferroni‐corrected p values by conducting nonparametric Mann–Whitney U tests.
Results
Both GBSS and ROI analysis detected diffused GM microstructural alterations in CBS and PSP patients as indexed by changes in DTI and FWI measures (Figures 3 and 4). GM microstructural changes in the frontoparietal lobe, including the precentral and postcentral gyrus, were more severe in CBS patients than in PSP patients. In contrast, there was no difference detected in GM microstructural changes in the thalamus between CBS and PSP. VBM analysis also detected a significantly decreased GM volume only in the bilateral precentral gyrus in CBS patients relative to the HC.Discussion
We used DTI, FWI, and VBM analyses to examine the cortical and subcortical structures in patients with CBS and PSP and healthy controls. Diffuse abnormalities in DTI and FWI parameters were detected within the GM of the CBS and PSP patients, whereas only VBM detected a significantly decreased GM volume in the bilateral precentral gyrus in CBS patients relative to the HC. CBS patients had more severe changes in the cortical microstructure, such as the precentral gyrus and postcentral gyrus, whereas no significant changes were detected in the thalamus. Both CBS and PSP are degenerative diseases with tau deposition in the brain, but CBS is known to have more severe tau deposition in the GM, especially in the precentral and postcentral gyrus, 8 and our research results are in good agreement with the findings of the pathological study. In addition, it was reported that tau deposition in the thalamus is nearly identical between CBS and PSP, 8 unlike other GM regions, which is also consistent with our results.Conclusion
We showed that DTI and FWI can detect cerebral GM abnormalities, which likely reflects tau-related pathology in CBS and PSP patients more sensitively than conventional VBM analysis.Acknowledgements
Data used in the preparation of this article were obtained from the 4-Repeat Tauopathy Neuroimaging Initiative (4RTNI; http://4rtni-ftldni.ini.usc.edu) and FTLDNI (http://4rtni-ftldni.ini.usc.edu/) database. The investigators at 4RTNI and FTLDNI contributed to the design and implementation of 4RTNI and FTLDNI and/or provided data but did not participate in the analysis or writing of this report. Data collection and sharing for this project were funded by the 4RTNI (National Institutes of Health grant R01 AG038791) and through generous contributions from the Tau Research Consortium. The study is coordinated through the University of California, San Francisco, Memory and Aging Center. 4RTNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California. Data collection and sharing for this project were funded by the Frontotemporal Lobar Degeneration Neuroimaging Initiative (National Institutes of Health grant R01 AG032306). This study was coordinated through the University of California, San Francisco, Memory and Aging Center. FTLDNI data were disseminated by the Laboratory for Neuro Imaging at the University of Southern California.References
- Litvan I, Bhatia KP, Burn DJ, et al. Movement Disorders Society Scientific Issues Committee report: SIC Task Force appraisal of clinical diagnostic criteria for Parkinsonian disorders. Movement disorders: official journal of the Movement Disorder Society 2003;18:467–486.]
- Paviour DC, Price SL, Jahanshahi M, Lees AJ, Fox NC. Longitudinal MRI in progressive supranuclear palsy and multiple system atrophy: rates and regions of atrophy. Brain: a journal of neurology 2006;129:1040–1049.
- Whitwell JL, Jack CR, Jr., Parisi JE, et al. Rates of cerebral atrophy differ in different degenerative pathologies. Brain: a journal of neurology 2007;130:1148–1158.
- Kamagata K, Zalesky A, Hatano T, et al. Gray Matter Abnormalities in Idiopathic Parkinson's Disease: Evaluation by Diffusional Kurtosis Imaging and Neurite Orientation Dispersion and Density Imaging. Human brain mapping 2017.
- Pasternak O, Sochen N, Gur Y, Intrator N, Assaf Y. Free water elimination and mapping from diffusion MRI. Magnetic resonance in medicine 2009;62:717–730.
- Ball G, Srinivasan L, Aljabar P, et al. Development of cortical microstructure in the preterm human brain. Proceedings of the National Academy of Sciences of the United States of America 2013;110:9541–9546.
- Desikan RS, Segonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 2006;31:968-980.
- Kouri N, Murray ME, Hassan A, et al. Neuropathological features of corticobasal degeneration presenting as corticobasal syndrome or Richardson syndrome. Brain: a journal of neurology 2011;134:3264–3275.
Figures
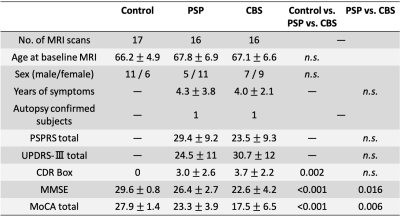
Figure 1. Demographic and clinical characteristics.
CBS, corticobasal syndrome; CDR Box, Clinical Dementia Rating Sum of Boxes; MMSE, Mini-Mental State Examination; Moca, Montreal Cognitive Assessment; PSP, progressive supranuclear palsy; PSPRS, PSP rating scale; UPDRS-III, Part-III (motor exams) of the Unified Parkinson's Disease Rating Scale.
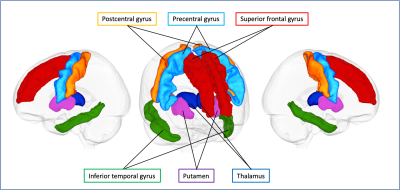
Figure 2. Gray matter regions of interest.
Six gray matter regions, which are described in the previous literature as vulnerable to tau pathology, based on the Desikan–Killiany cortical and subcortical atlas are shown in one representative case.
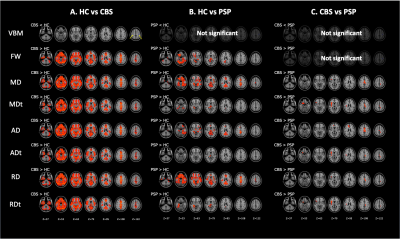
Figure 3. Comparison of VBM, DTI (MD, AD, and RD), and FW imaging (MDt, ADt, RDt, and FW) indices between patients with CBS and PSP and healthy controls.
The results showed reduced gray matter volume (blue–right blue voxels) and increased FW, MD, MDt, AD, ADt, RD, and RDt (red–yellow voxels) in CBS patients relative to controls (A). GBSS results showed increased FW, MD, MDt, AD, ADt, RD, and RDt (red–yellow voxels) in PSP patients relative to controls (B). GBSS results showed increased MD, MDt, AD, ADt, RD, and RDt (red–yellow voxels) in CBS patients relative to PSP patients (C).
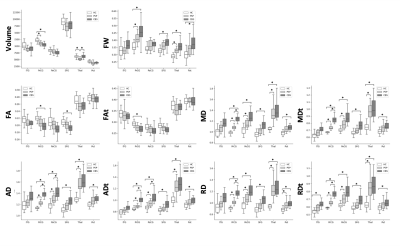
Figure 4. Box plots of diffusion MRI parameters in six gray matter regions (ITG, PrCG, PoCG, SFG, Thal, and Put) previously described as vulnerable to tau pathology.
Plots indicate median values, and boxes include the upper and lower quartiles.
ITG, inferior temporal gyrus; PoCG, postcentral gyrus; PrCG, precentral gyrus; Put, putamen; SFG, superior frontal gyrus; Thal, thalamus.