2053
Harmonization of multi-site diffusion MRI data of the Adolescent Brain Cognitive Development (ABCD) Study1Brigham and Women's Hospital and Harvard Medical School, Boston, MA, United States, 2Brigham and Women's Hospital, Boston, MA, United States, 3Isomics, Boston, MA, United States
Synopsis
This study presents our harmonization efforts on the multi-site diffusion MRI data of the ~12,000 adolescents from the Adolescent Brain Cognitive Development (ABCD) study, collected from 22 sites using Siemens, GE and Philips scanners. On the minimally preprocessed diffusion MRI data provided by the ABCD study - release 3, we first applied brain masking using our deep learning tool which showed 99% Dice overlap performance compared to the manually corrected masks. Next, we harmonized the dMRI data from 22 sites (with 45 scanner settings) using our previously-validated software. The harmonized diffusion MRI data will be shared through the NIMH data archive.
Introduction
The Adolescent Brain Cognitive Development (ABCD) study1,2 is the largest long-term study of brain development and child health in the United States. This consortium has collected neuroimaging, genetic, and behavioral data from 22 sites across the country3 for ~12,000 children ages 108-131 months. Although the study ensured similar imaging protocols across sites, it is not advisable to naively pool these datasets from all sites for joint analysis without additional processing4. This is because scanner-related differences can induce significant bias, especially in diffusion MRI (dMRI) data5. This bias can be due to different hardware specifications, different acquisition settings, and/or different software versions. Thus, the dMRI data can vary not only between vendors but even between identical scanners running different software versions6. The slightest scanner differences therefore can add large unwanted scanner-related effects to the dMRI data, thereby dramatically reducing statistical power. Taking steps to remove these scanner-related effects is thus critically important for neuroimaging studies of psychiatric disorders, where the effect sizes of the group differences related to biology or disease are usually very small7. This abstract presents our preprocessing and harmonization strategies on multi-site dMRI data of the ABCD study as part of the NIH R01MH119222 (PIs: Rathi, O’Donnell).Methods
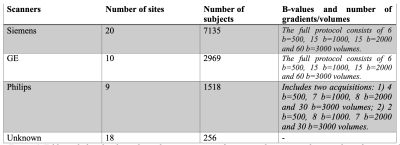
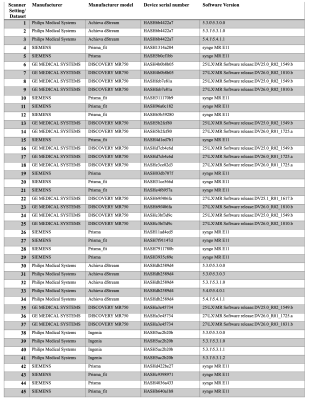
Dataset.We worked on the baseline scans of the minimally preprocessed dMRI data from the ABCD study (release 3). Diffusion MRI data was acquired using 3T Siemens, Philips, and GE scanners (Figure 1) from 22 sites using 45 different scanner-related settings in total (Figure 2). Minimal preprocessing steps included: eddy-current correction, motion correction, B0 inhomogeneity correction, gradient unwarp, replacement of bad slice-frames, between scan motion correction, rigid body registration to the atlas, and resampling to 1.7mm^3. See Hatton et al.8 for more details of the preprocessing.
Brain Masking.
Data made available does not provide brain masks, which is a prerequisite for our harmonization algorithm. Therefore, we developed a deep learning-based brain masking tool that robustly extracts the brain from dMRI data in a few seconds on a GPU (https://github.com/pnlbwh/CNN-Diffusion-MRIBrain-Segmentation). The network was trained on the dMRI data of 1500 subjects which were collected from multiple clinical and non-clinical datasets. It was tested and manually quality controlled over a thousand unseen subjects from other studies as well as on the ABCD data. We deployed the brain masking in the ABCD dMRI data of 11,622 subjects.
Harmonization.
We next applied our retrospective harmonization algorithm4. Our harmonization approach (https://github.com/pnlbwh/dMRIharmonization), based on rotation invariant spherical harmonics (RISH), is applied at the signal level after the minimal preprocessing to remove the scanner differences across multi-site dMRI data, while accounting for non-linearities both in the dMRI signal itself and the scanner bias4. After harmonization, any model fitting or connectivity analysis can be applied, unlike other approaches that only harmonize diffusion measures9. The performance of this algorithm was also validated on multiple datasets, including a large schizophrenia database10 as well as in the harmonization community challenge11. Since each site had scanner upgrades or used multiple scanners during the study, we, therefore, applied the harmonization across all 45 scanner-related settings, each of which we term as “dataset” henceforward (Figure 3). In the first step of the harmonization, we selected one dataset as the reference from 45 datasets. The rest of the 44 datasets are referred to as target datasets. A subset of 30 healthy subjects, who were age- and sex-matched at the group level across each target and reference dataset, were selected to learn the scanner differences using RISH features. After that, the learned mappings across RISH features were applied to the entire dMRI data of the corresponding target dataset.
Results
Brain Masking.The average performance of the algorithm over the unseen thousand subjects, which was not part of the training (of note, ABCD study was not part of this analysis), is over 97% for the Dice overlap score. In the ABCD study, the performance increased to ~99% (compared to manually curated masks). This is most probably due to the fact that 100 dMRI data from the ABCD study was part of our training set (Figure 3).
Harmonization.
As noted, we selected one dataset as a reference, and all other datasets (from the rest of the 44 datasets) were selected as target datasets to be harmonized to the reference independently. Refer to Figure 4, where we demonstrated the performance of the harmonization for two datasets (dataset 1 and dataset 2). To evaluate the performance of the harmonization, average fractional anisotropy along the whole brain white matter was computed for the reference, as well as target datasets before and after harmonization. Unpaired t-tests showed large differences between reference and target datasets (p=0.0017;t=3.52) prior to harmonization. Statistical differences across datasets were removed after harmonization (p=0.53;t=1.15).
Discussion and Conclusion
We presented our brain masking and harmonization efforts on the dMRI data of the ABCD study, collected from 22 sites with 45 datasets, where the harmonized data will be shared in the NIMH data archive. This will allow large-scale data analysis as if the data came from the same scanner, which should significantly increase statistical power with the ability to better characterize the neurodevelopmental changes in the white matter of adolescents.Acknowledgements
The authors have been supported by the following grant: NIH R01 MH119222 (Rathi, O'Donnell). The project also acknowledges that the research has partly been supported by the BWH Program for Interdisciplinary Neuroscience, through a gift from Lawrence and Tiina Rand (Cetin-Karayumak).References
1. Volkow, N. D. et al. The conception of the ABCD study: From substance use to a broad NIH collaboration. Dev. Cogn. Neurosci. 32, 4–7 (2018).
2. Lisdahl, K. M. et al. Adolescent brain cognitive development (ABCD) study: Overview of substance use assessment methods. Dev. Cogn. Neurosci. 32, 80–96 (2018).
3. Casey, B. J. et al. The Adolescent Brain Cognitive Development (ABCD) study: Imaging acquisition across 21 sites. Dev. Cogn. Neurosci. 32, 43–54 (2018).
4. Cetin Karayumak, S. et al. Retrospective harmonization of multi-site diffusion MRI data acquired with different acquisition parameters. Neuroimage 184, 180–200 (2019).
5. Landman, B. A. et al. Effects of diffusion weighting schemes on the reproducibility of DTI-derived fractional anisotropy, mean diffusivity, and principal eigenvector measurements at 1.5T. NeuroImage vol. 36 1123–1138 (2007).
6. Helmer, K. G. et al. Multi-site Study of Diffusion Metric Variability: Characterizing the Effects of Site, Vendor, Field Strength, and Echo Time using the Histogram Distance. Proc. SPIE Int. Soc. Opt. Eng. 9788, (2016).
7. White, T. et al. Global white matter abnormalities in schizophrenia: a multisite diffusion tensor imaging study. Schizophr. Bull. 37, 222–232 (2011).
8. Hatton, S. Preview of the Adolescent Brain Cognitive Development (ABCD) Study Release 3.0. Biological Psychiatry vol. 87 S110–S111 (2020).
9. Fortin, J.-P. et al. Harmonization of multi-site diffusion tensor imaging data. Neuroimage 161, 149–170 (2017).
10. Cetin-Karayumak, S. et al. White matter abnormalities across the lifespan of schizophrenia: a harmonized multi-site diffusion MRI study. Mol. Psychiatry 25, 3208–3219 (2020).
11. Ning, L. et al. Cross-scanner and cross-protocol multi-shell diffusion MRI data harmonization: Algorithms and results. NeuroImage vol. 221 117128 (2020).
Figures
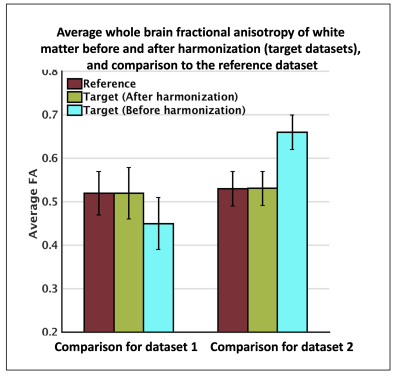
Harmonization performance on two datasets: one dataset was selected as a reference, two other datasets (from the rest of the 44) were selected as target (dataset 1, dataset 2) to be harmonized to the reference. To evaluate the performance of the harmonization, average fractional anisotropy along the whole brain white matter was computed for the reference, as well as target datasets before and after harmonization. Unpaired t-tests showed large between-dataset differences (p=0.0017) prior to harmonization. Statistical differences were removed after harmonization (p=0.53).