2052
More than just axons: A positive relationship between an intracellular isotropic diffusion signal & pubertal development in white matter regions1Department of Radiology and Medical Imaging, University of Virginia, Charlottesville, VA, United States, 2Brain Institute, University of Virginia, Charlottesville, VA, United States, 3Department of Public Health Sciences, University of Virginia, Charlottesville, VA, United States
Synopsis
Understanding how the brain develops during adolescence is important for evaluating neuronal developments that affect mental health throughout the lifespan. This study uses 3-tissue constrained spherical deconvolution (3T-CSD) to examine the relationship between brain diffusion microstructure in deep white matter ROIs and pubertal development in a cross-sectional group of 4752 adolescents. An anisotropic diffusion signal fraction was found to have a negative correlation, while an intracellular isotropic diffusion signal fraction had a positive correlation with pubertal development across the majority of axonal ROIs. These results provide evidence for complex microstructural changes in brain development within the white matter skeleton.
Introduction
In adolescent development, puberty is a critical period involving extensive physical, behavioral, and neurological changes that can have lifelong consequences for health and well-being1,2. Previous neuroimaging studies, including those focused on microstructure, have largely focused on axonal changes3,4,5, and have consistently described increases in the global development and volume of white matter (WM)6,7,8. However the WM regions of the brain contain numerous glial cells varieties and extracellular space that contributes to a diverse diffusion profile9. In this study we utilize a method to measure signal contributions from axonal (WM-like), intracellular (GM-like), and extracellular (CSF-like) tissue compartments and present evidence for a widespread positive relationship between an isotropic, intracellular, diffusion signal within the WM skeleton and pubertal stage. This may reflect changes in glial activity, or apoptotic events from neuronal pruning9.Methods
Unprocessed dMRI images were obtained from the Adolescent Brain Cognitive Development (ABCD) study, a multi-site longitudinal study of neuroimaging and cognition beginning at 9-10 years of age through adolescence10. Baseline images were acquired with a multiband accelerated sequence that had an isotropic voxel size 1.7 x 1.7 x 1.7 mm3 with TE = 88 ms and TR = 4100 ms. Using a multi-shell protocol 7 images were acquired at b= 0, 6 directions were acquired at b=500 s/mm2, 15 directions were acquired at both b=1000 s/mm2 and at b=2000 s/mm2, and 60 directions were acquired at b=3000 s/mm2. Only images acquired using the Siemens Prisma 3T platform were analyzed to avoid manufacturer and sequence differences, including field gradient strength, TE, and TR, that have previously been demonstrated to affect outcome 3T-CSD signal fraction results.To achieve better contrast between tissue ROIs11, each subject was analyzed using SS3T-CSD12 implemented on the b=3000 s/mm2 shell as available in MRtrix3Tissue (https://3tissue.github.io/), a fork of MRtrix313. Several preprocessing steps utilized FSL14. Diffusion images were denoised15, corrected for Gibbs ringing16, susceptibility distortions17, motion18, and eddy currents19 then voxels were upsampled to 1.3 mm isotropic20. Average response functions were generated for WM-, GM-, and CSF-like tissues from the images21 and the fiber orientation distribution (FOD) calculated for each voxel12. 3-tissue signal fractions were calculated from the FODs11.
A cohort specific template was constructed from a random selection of 50 subjects’ WM-FODs using symmetric diffeomorphic registration of the FODs themselves. Each subject was then individually registered alongside a b-value matched version of the NTU-DSI-122 template to allow the 48 ROIs from the ICBM-DTI-81 template (JHU-DTI atlas22,23,24) to be moved into the cohort template25. Quality control was performed manually on the final subject images in template space which resulted in a final cohort of 4752 subjects (2256 female). Pubertal development was assessed via the pubertal development scale score (PDSS)26, a parental survey of physical changes in adolescent secondary sex characteristics. A linear regression model fitted using ordinary least squares was utilized to predict PDSS from the signal fraction in each of the 3 tissue compartments, in each of the 48 JHU-DTI ROIs, controlled for subject age, sex, handedness, and total brain volume with a Benjamini and Hochberg false discovery threshold.
Results
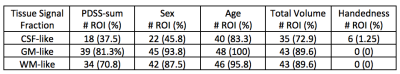
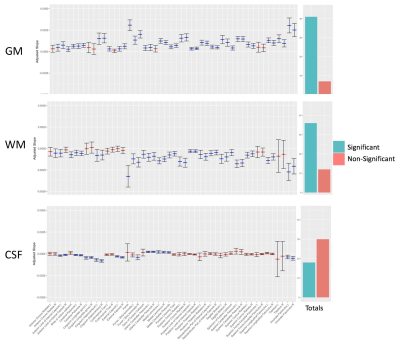
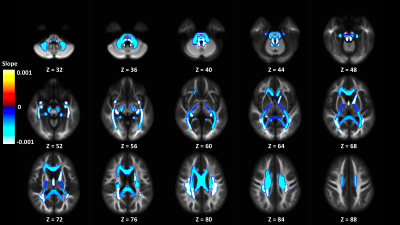
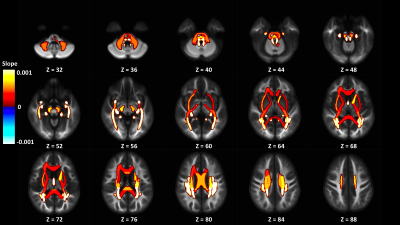
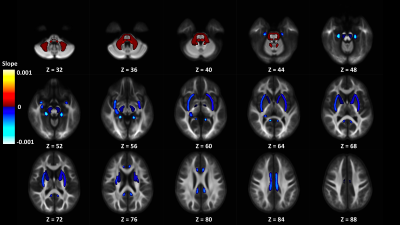
Out of 144 total linear models, tissue signal fraction was significantly associated with PDSS measures of pubertal development in 91 ROIs (~63%; for a summary of variable associations see Table 1). Most notably, in all of the GM-like signal fraction ROIs that were significant (39 of 48, 81.3%) there was a positive association with pubertal development as measured by PDSS. In all of the significant WM-like signal fraction ROIs (34 of 48, 70.8%) there was a negative association with PDSS. There was not a consistent direction of association between CSF-like and PDSS in significant ROIs (18 of 48, 37.5%). The directions of each signal fraction association, as well as the significance of each ROI, is displayed in Figure 1. The location of each significant ROI, as well as the slope of the association between PDSS and signal fraction for WM-, GM-, and CSF-like is displayed in Figures 2, 3, and 4, respectively.Discussion
This study provides evidence for microstructural changes in brain tissue beyond anisotropic diffusion signal typically associated with WM axon fibers. Initially, the findings here seem to contradict established neuroimaging literature. Recent dMRI work using fixel-based analysis, which also relies on CSD techniques, has found that WM fibers mature in ROIs from the JHU-DTI atlas in response to pubertal development measured using the same scale applied here3,4. However it is important to note that the 3T-CSD technique measures relative levels of each tissue compartment and does not exclude the possibility WM fibers also mature in response to pubertal development. Possible physiological interpretations of this effect include changes in the number or activity of glial cells, such as oligodendrocytes responsible for increased myelin27, or apoptotic events that are thought to occur during neuronal pruning, which may contribute to an increased intracellular isotropic signal via the breakup of axons and their consumption by glial cells28.Conclusion
In this cross-sectional dMRI study of 4752 adolescents from the baseline collection of the ABCD study we have identified a relationship between physical manifestations of pubertal status and several measurements of brain tissue microstructure across a widespread number of primarily axonal brain regions.Acknowledgements
No acknowledgement found.References
1. Lerner, R. M. & Steinberg, L., (2009). Handbook of adolescent psychology, volume 1: Individual bases of adolescent development. s.l.:John Wiley & Sons.
2. Juraska, J. M. & Willing, J., (2017). Pubertal onset as a critical transition for neural development and cognition. Brain Research, Volume 1654, pp. 87-94.
3. Genc, S., Seal, M. L., Dhollander, T., Malpas, C. B., Hazell, P., & Silk, T. J. (2017). White matter alterations at pubertal onset. NeuroImage, 156, 286-292.
4. Genc, S., Malpas, C. B., Gulenc, A., Sciberras, E., Efron, D., Silk, T. J., & Seal, M. L. (2020). Longitudinal patterns of white matter fibre density and morphology in children are associated with age and pubertal stage. Developmental cognitive neuroscience, 45, 100853.
5. Barendse, M. E., Simmons, J. G., Smith, R. E., Seal, M. L., & Whittle, S. (2020). Adrenarcheal hormone-related development of white matter during late childhood. NeuroImage, 223, 117320.
6. Peper, J. S., Pol, H. H., Crone, E. A. & Van Honk, J., (2011). Sex steroids and the brain structure in pubertal boys and girls: a mini-review of neuroimaging studies. Neuroscience, Volume 191, pp. 28-37.
7. Lebel, C. & Beaulieu, C., (2011). Longitudinal development of human brain wiring continues from childhood into adulthood. Journal of Neuroscience, Volume 31, pp. 10937-10947.
8. Lebel, C. & Deoni, S., (2018). The development of brain white matter microstructure. NeuroImage, Volume 182, pp. 207-218.
9. Nave, K. A. (2010). Myelination and support of axonal integrity by glia. Nature, 468(7321), 244-252.
10. Volkow, N. D., Koob, G. F., Croyle, R. T., Bianchi, D. W., Gordon, J. A., Koroshetz, W. J., ... & Deeds, B. G. (2018). The conception of the ABCD study: From substance use to a broad NIH collaboration. Developmental cognitive neuroscience, 32, 4-7.
11. Newman, B. T., Dhollander, T., & Druzgal, T. J., (2020). Single-shell derived tissue signal fraction maps show increased contrast between hippocampal subfields compared to multi-shell analysis. 28th Annual International Meeting Society of Magnetic Resonance in Medicine, 28, 4424
12. Dhollander, T., & Connelly, A. (2016). A novel iterative approach to reap the benefits of multi-tissue CSD from just single-shell (+ b= 0) diffusion MRI data. In Proc ISMRM (Vol. 24, p. 3010).
13. Tournier, J. D., Smith, R., Raffelt, D., Tabbara, R., Dhollander, T., Pietsch, M., ... & Connelly, A. (2019). MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. NeuroImage, 202, 116137.
14. Smith, S. M., Jenkinson, M., Woolrich, M. W., Beckmann, C. F., Behrens, T. E., Johansen-Berg, H., ... & Niazy, R. K. (2004). Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage, 23, S208-S219.
15. Veraart, J., Novikov, D. S., Christiaens, D., Ades-Aron, B., Sijbers, J., & Fieremans, E. (2016). Denoising of diffusion MRI using random matrix theory. Neuroimage, 142, 394-406.
16. Kellner, E., Dhital, B., Kiselev, V. G., & Reisert, M. (2016). Gibbs‐ringing artifact removal based on local subvoxel‐shifts. Magnetic resonance in medicine, 76(5), 1574-1581.
17. Andersson, J. L., Skare, S., & Ashburner, J. (2003). How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage, 20(2), 870-888.
18. Andersson, J. L., Graham, M. S., Zsoldos, E., & Sotiropoulos, S. N. (2016). Incorporating outlier detection and replacement into a non-parametric framework for movement and distortion correction of diffusion MR images. Neuroimage, 141, 556-572.
19. Andersson, J. L., & Sotiropoulos, S. N. (2016). An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage, 125, 1063-1078.
20. Greenspan, H. (2009). Super-resolution in medical imaging. The computer journal, 52(1), 43-63.
21. Dhollander, T., Mito, R., Raffelt, D., & Connelly, A. (2019). Improved white matter response function estimation for 3-tissue constrained spherical deconvolution. In Proc. Intl. Soc. Mag. Reson. Med (Vol. 555).
22. Mori, S., Wakana, S., Van Zijl, P. C., & Nagae-Poetscher, L. M. (2005). MRI atlas of human white matter. Elsevier.
23. Wakana, S., Caprihan, A., Panzenboeck, M. M., Fallon, J. H., Perry, M., Gollub, R. L., ... & Blitz, A. (2007). Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage, 36(3), 630-644.
24. Hua, K., Zhang, J., Wakana, S., Jiang, H., Li, X., Reich, D. S., ... & Mori, S. (2008). Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage, 39(1), 336-347.
25. Newman, B. T., Untaroiu, A., & Druzgal, T. J. A novel diffusion registration method with the NTU-DSI-122 template to transform free water signal fraction maps to stereotaxic space. 28th Annual International Meeting Society of Magnetic Resonance in Medicine, 28, 4541.
26. Shirtcliff, E. A., Dahl, R. E., & Pollak, S. D. (2009). Pubertal development: correspondence between hormonal and physical development. Child development, 80(2), 327-337.
27. Paus, T. (2010). Growth of white matter in the adolescent brain: myelin or axon?. Brain and cognition, 72(1), 26-35.
28. Willing, J., & Juraska, J. M. (2015). The timing of neuronal loss across adolescence in the medial prefrontal cortex of male and female rats. Neuroscience, 301, 268-275.
Figures