2045
White matter microstructure associated with functional connectivity changes following short-term learning of a visuomotor sequence1Physics, Concordia University, Montreal, QC, Canada, 2Montreal Heart Institute, Montreal, QC, Canada, 3Neurology, Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany, 4Clinic for Cognitive Neurology, Leipzig, Germany, 5Leipzig University Medical Centre, IFB Adiposity Diseases, Leipzig, Germany, 6Collaborative Research Centre 1052-A5, University of Leipzig, Leipzig, Germany, 7Biomedical Engineering, McGill University, Montreal, QC, Canada, 8Montreal Neurological Institute, Montreal, QC, Canada, 9Faculty of Social and Behavioral Sciences, University of Amsterdam, Amsterdam, Netherlands, 10Psychology, Concordia University, Montreal, QC, Canada
Synopsis
To characterize the temporal dynamics of plasticity, we conducted a longitudinal MRI study at ultra-high field (7T) during the learning process of a sequential visuomotor task, in a learning and control group. WM microstructure was altered in the tracts underlying the primary motor and sensorimotor cortices, and in tracts adjacent to the right supplementary motor area (SMA), where changes in functional connectivity were also found in this cohort. Our study provides evidence for short-term white matter plasticity in the sensorimotor network, where the SMA would play a key role in linking the spatial and motor aspects of motor sequence learning.
Introduction
Efficient neural transmission is crucial for optimal brain function, yet the plastic potential of white matter (WM) has long been overlooked 1–3. Growing evidence now shows that modifications to axons and myelin occur not only as a result of long-term learning, but also after short training periods 4–8. As sequential behaviors are part of nearly every human function, studying the neural correlates of motor sequence learning (MSL) provides a highly valuable window into how the brain can be reshaped through learning and how these alterations then support new functions. MSL occurs in overlapping learning stages (i.e., initial fast stage, slow subsequent stage and retention) and different neural circuits are involved in each stage 9–12. However, few studies investigating WM plasticity have characterized stage-specific changes in microstructure as several studies either use cross-sectional 4–6 or pre-post longitudinal designs 7,8.Methods
In this study, 40 healthy right-handed subjects performed a sequential pinch-force task (SPFT) 13,14 with their right hand on 5 consecutive days: inside the MRI scanner on days 1 (d1), 2 (d2), and 5 (d5), and outside the scanner on d3 and d4. The task was also performed inside the scanner at a retention session, 12 days after cessation of training (d17). Twenty subjects learned a complex visuomotor sequence (LRN) and 20 performed a simple sequence (SMP). The control condition (SMP) allowed to distinguish structural alterations related to motor execution from those that are specific to sequence learning. DWI data were acquired from an EPI sequence (TR/TE=10100/62.8ms, 1.2mm isotropic, b=1000s/mm2, 20 directions) with simultaneous multi-slice imaging in a Siemens 7T scanner. Resting-state fMRI data were acquired with a blood-oxygen-level-dependent (BOLD) sequence (TR/TE= 1130/22ms, 1.2mm isotropic, flip angle= 40°). DWI data were preprocessed and then fitted to a tensor model (DTI) to compute maps of fractional anisotropy (FA), medial, axial and radial diffusivities (MD, AD and RD) 15-17. Voxel-wise analyses were conducted on DTI metrics maps within a WM mask, using a flexible factorial design in SPM. This allowed the identification of WM regions involved in different learning stages. To relate structural changes in WM to functional changes, ROIs were defined from results of similar voxel-wise analyses in functional connectivity metrics (i.e., Eigenvector and degree centrality; EC and DC), assessed by our group using resting-state fMRI data (unpublished). ROIs were generated from rs-fMRI clusters in the right supplementary motor area (SMA), bilateral superior parietal cortex (SPC), right pars opercularis (R PO), and right globus pallidus (GP) by inflating ROIs in the underlying WM. Repeated-measures ANOVAs were conducted on the mean DTI values within those regions.Results
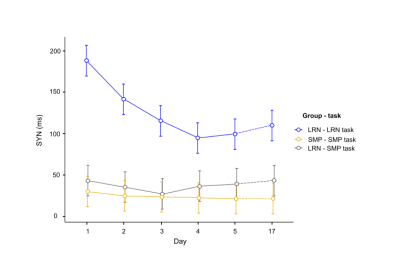
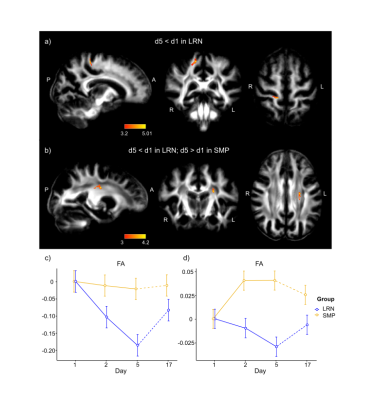
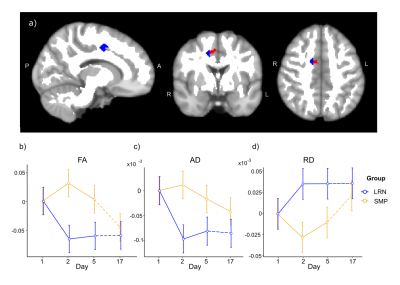
Consistent with behavioral results, where most improvements in temporal synchronization (SYN) occurred between the two first days in the LRN group (Fig. 1), structural changes in WM were observed only in the early phase of learning (d1-d2), and over the entire learning period (overall learning; d1-d5). WM microstructure was altered in the tracts underlying the primary motor and sensorimotor cortices (M1 and S1) during the overall learning period (Fig. 2). FA in the left corticospinal tract (CST) inferior to M1 was found to decrease in the LRN group, while it increased in the SMP group (t= 4.20, 𝘱FWE= 0.002). FA also decreased in the right ascending sensorimotor tract (SMT) adjacent to S1 in the LRN group (t= 5.01, 𝘱FWE= 0.005). Moreover, our structural findings in WM were spatially related to changes in functional connectivity. WM microstructure was altered during the early phase of learning (d1-d2) in the ROI underlying the right SMA (Fig. 3), where a sequence-specific decrease in functional connectivity was found during overall learning in this cohort (unpublished). Significant decreases in FA and AD (F(2, 36)= 5.82, p=0.006; F(1.38, 24.91)= 6.27, p=0.012, respectively), as well as an increase in RD (F(1.44, 25.99)= 3.93, p= 0.044), across time were found in this ROI.Discussion
Decreased FA in fiber tracts connecting to the right S1 during overall learning may reflect suppression of activity in S1 ipsilateral to the hand used in the SPFT, which may contribute in enhancing processing of task-relevant inputs by the contralateral S1 18,19. The slow decrease in FA in WM tracts underlying M1 is consistent with other studies in which activity in M1 was shown to progressively decrease as motor learning progresses, perhaps reflecting enhanced network efficiency 9,20–22. Similarly, we hypothesized that the decreases in FA and AD in the SMA ROI, and decreased functional connectivity in this region, which is known for its role in sequence processing, reflected a reduced need for resources to plan and coordinate movements as a skill is mastered 23–25.Conclusion
Together, our findings provide evidence for highly dynamic WM plasticity in the sensorimotor network during short-term MSL, where the SMA would play a key role in linking the spatial and motor aspects of motor sequence learning 9,26. Interestingly, the changes that were revealed in WM microstructure paralleled the functional changes in connectivity that were found in the same cohort, indicating a potential link between structural and functional plastic processes. A better understanding of how learning can structurally shape neural networks could have important implications in other fields of research such as in stroke rehabilitation, to optimize interventions through motor learning.Acknowledgements
We would like to thank Elisabeth Wladimirov and Domenica Wilfling for their help and involvement in data acquisition and logistics of the multi-modal plasticity initiative (MMPI) dataset. This work was supported by the Max Planck Society, the MaxNetAging Research School (Max Planck Institute for Demographic Research, ATJ), the NWO Vici grant (PI: Birte Forstmann)(PLB), the National Science and Engineering Research Council (NSERC; CJG: RGPIN 2015-04665, CJS: RGPIN-2020-06812, DGECR-2020-00146), the Michal and Renata Hornstein Chair in Cardiovascular Imaging (CJG), the Heart and Stroke Foundation (New Investigator Award, CJG and CJS), the Canadian Institutes of Health Research (HNC 170723), the Fonds de Recherche du Québec - Nature et Technologies (CJS and JH), the Fonds de Recherche du Québec - Santé (JH), and the Réseau de Recherche en Santé cardiométabolique, diabète et obésité (CMDO; JH).References
1. Fields RD. A new mechanism of nervous system plasticity: activity-dependent myelination. Nat Rev Neurosci. 2015 Dec;16(12):756–67.2.
2. Sampaio-Baptista C, Johansen-Berg H. White matter plasticity in the adult brain. Neuron. 2017;96(6):1239–1251.3.
3. Waxman SG. Integrative properties and design principles of axons. Int Rev Neurobiol. 1975;18:1–40.4.
4. Giacosa C, Karpati FJ, Foster NEV, Penhune VB, Hyde KL. Dance and music training have different effects on white matter diffusivity in sensorimotor pathways. NeuroImage. 2016 15;135:273–86.5.
5. Giacosa C, Karpati FJ, Foster NEV, Hyde KL, Penhune VB. The descending motor tracts are different in dancers and musicians. Brain Struct Funct. 2019 Dec;224(9):3229–46.6.
6. Hänggi J, Koeneke S, Bezzola L, Jäncke L. Structural neuroplasticity in the sensorimotor network of professional female ballet dancers. Hum Brain Mapp. 2010;31(8):1196–206.7.
7. Hofstetter S, Tavor I, Moryosef ST, Assaf Y. Short-Term Learning Induces White Matter Plasticity in the Fornix. J Neurosci. 2013 Jul 31;33(31):12844–50.8.
8. Scholz J, Klein MC, Behrens TE, Johansen-Berg H. Training induces changes in white-matter architecture. Nat Neurosci. 2009;12(11):1370–1371.9.
9. Dayan E, Cohen LG. Neuroplasticity subserving motor skill learning. Neuron. 2011 Nov 3;72(3):443–54.10.
10. Halsband U, Lange RK. Motor learning in man: a review of functional and clinical studies. J Physiol-Paris. 2006;99(4–6):414–424.11.
11. Luft AR, Buitrago MM. Stages of motor skill learning. Mol Neurobiol. 2005 Dec 1;32(3):205–16.12.
12. Penhune VB, Steele CJ. Parallel contributions of cerebellar, striatal and M1 mechanisms to motor sequence learning. Behav Brain Res. 2012;226(2):579–591.13.
13. Camus M, Ragert P, Vandermeeren Y, Cohen LG. Mechanisms controlling motor output to a transfer hand after learning a sequential pinch force skill with the opposite hand. Clin Neurophysiol. 2009 Oct 1;120(10):1859–65.14.
14. Krakauer JW, Hadjiosif AM, Xu J, Wong AL, Haith AM. Motor Learning. Compr Physiol. 2019 14;9(2):613–63.15.
15. Avants, Tustison N, Johnson H. Advanced Normalization Tools (ANTS). 2009;41.16.
16. Tournier J-D, Smith R, Raffelt D, Tabbara R, Dhollander T, Pietsch M, et al. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. NeuroImage. 2019;116137.17.
17. Andersson JL, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. 2016;125:1063–1078.
18. Kastrup A, Baudewig J, Schnaudigel S, Huonker R, Becker L, Sohns JM, et al. Behavioral correlates of negative BOLD signal changes in the primary somatosensory cortex. NeuroImage. 2008 Jul 15;41(4):1364–71.16.
19. Staines WR, Graham SJ, Black SE, McIlroy WE. Task-Relevant Modulation of Contralateral and Ipsilateral Primary Somatosensory Cortex and the Role of a Prefrontal-Cortical Sensory Gating System. NeuroImage. 2002 Jan 1;15(1):190–9.17.
20. Costa RM, Cohen D, Nicolelis MAL. Differential Corticostriatal Plasticity during Fast and Slow Motor Skill Learning in Mice. Curr Biol. 2004 Jul 13;14(13):1124–34.18.
21. Poldrack RA. Imaging brain plasticity: conceptual and methodological issues—a theoretical review. Neuroimage. 2000;12(1):1–13.19.
22. Seidler RD, Purushotham A, Kim S-G, Ugurbil K, Willingham D, Ashe J. Neural correlates of encoding and expression in implicit sequence learning. Exp Brain Res. 2005 Aug 1;165(1):114–24.20.
23. Bassett DS, Yang M, Wymbs NF, Grafton ST. Learning-induced autonomy of sensorimotor systems. Nat Neurosci. 2015 May;18(5):744–51.21.
24. Finc K, Bonna K, He X, Lydon-Staley DM, Kühn S, Duch W, et al. Dynamic reconfiguration of functional brain networks during working memory training. Nat Commun. 2020 May 15;11(1):2435.22.
25. Mohr H, Wolfensteller U, Betzel RF, Mišić B, Sporns O, Richiardi J, et al. Integration and segregation of large-scale brain networks during short-term task automatization. Nat Commun. 2016 Nov 3;7(1):13217.23.
26. Hikosaka O, Nakamura K, Sakai K, Nakahara H. Central mechanisms of motor skill learning. Curr Opin Neurobiol. 2002 Apr 1;12(2):217–22.
Figures