2043
Cognitive training-derived microstructural and functional neuroplasticity and the neural mechanisms underlying the far-transfer effect1Department of Rehabilitation Science, Hokkaido University, Sapporo, Japan, 2Department of Biomarker Imaging Science, Hokkaido University, Sapporo, Japan, 3Department of Medical Physics, Hokkaido University Hospital, Sapporo, Japan, 4Department of Rehabilitation, Hokkaido University Hospital, Sapporo, Japan, 5Global Center for Biomedical Science and Engineering, Hokkaido University Faculty of Medicine, Sapporo, Japan
Synopsis
Little is known about how microstructural and functional neuroplasticity occurs upon cognitive training and the relationship between cognitive training-derived brain changes and cognitive performance. This prospective study aimed to elucidate cognitive training-derived neuroplasticity and the mechanism underlying transfer effects using diffusion spectrum imaging (DSI) and resting-state functional MRI (rsfMRI). The results suggest that the right inferior parietal lobule and its neural connections and the right cerebellar vermis may modulate the far-transfer effect.
INTRODUCTION
Previous neuroimaging studies that employed DTI or functional MRI have reported that short-term training modulates the brain microstructure and function. Nevertheless, the relationship of these brain changes to cognitive performance has very little been explored. This prospective study aimed to elucidate the cognitive training-derived microstructural and functional neuroplasticity and the neural mechanisms underlying the far-transfer effect (improved performance of non-trained tasks that belong to other cognitive domains) of cognitive training through tests of correlation between the microstructural and functional brain changes as evaluated by MRI and the changes in cognitive performance.METHODS
This prospective study was approved by a local ethical committee, and written informed consent was obtained from all participants. Altogether 29 healthy subjects were included in this study. Each participant was assigned to either the training group or the control group. The training group included 21 subjects (mean age = 27.7 ± 6.0 years, nine women) and the control group 8 age, gender, and education level-matched subjects (mean age = 31.5 ± 9.5 years, four women). The training group underwent an hour-long cognitive training that included dual n-back task (DBT) and attention network task (ANT), five days a week for four weeks. Each group underwent a set of neuropsychological tests and brain MRI including diffusion spectrum imaging (DSI) (TR/TE= 9200/98 ms, voxel size= 2.5 × 2.5 × 2.5 mm3, bmax= 8000 s/mm2, 128 diffusion gradient directions) and resting-state functional MRI (rsfMRI) (TR/TE = 3000/30 ms, voxel size = 3.75 × 3.75 × 3.75mm3, 140 dynamic sessions) twice at an interval of 4-6 weeks. In the training group, these tests and MRI were performed before and after the training. The neuropsychological tests consisted of 5 tasks designed to reflect far-transfer effects of the assigned tasks. Generalized fractional anisotropy (GFA) and fractional anisotropy (FA) maps were generated from the DSI data, and resting-state functional connectivity (rsFC) was calculated from the rsfMRI data. The GFA and FA were analyzed voxel-by-voxel using the 2×2 (group × time) mixed-design ANOVA to identify the training-related brain microstructural changes (Figure 1). Uncorrected P< 0.001 for clusters containing at least 100 voxels were considered significant. Age, gender, and years of education, and cerebrospinal fluid (CSF) volumes were considered as covariates. The statistically significant clusters were then used as seeds to identify rsFC with any other brain regions through seed-to voxel analysis. FWE-corrected cluster-level P< 0.05 was considered statistically significant. Pearson’s product-moment correlation analyses were used to test the correlation between the training-derived changes in GFA, FA, and rsFC and neuropsychological test performance changes. Statistical significance was set as uncorrected P< 0.05.RESULTS
Neuropsychological test performance improved significantly in the training group after the cognitive training, which was evident in 4 of the five tasks (Figure 2). The mixed-design ANOVA revealed significant interaction of GFA and FA in the gray matter of the right inferior parietal lobule, which on post-hoc analysis revealed significantly decreased GFA and FA in the training group after the training (Figure 3). The significant GFA voxels had significant interaction with the training-induced decrease in rsFC of the right lateral occipital cortex (MNI coordinate x= 50, y=- 62, z= 44, cluster size= 91 voxels), the left inferior frontal gyrus (x= -48, y= 26, z= 18, cluster size= 90 voxels), and the frontal pole (x= 50, y=- 62, z= 44, cluster size= 91 voxels) (Figure 4). There were significant moderate negative correlations between the GFA changes in the right inferior parietal lobule and the changes in percent correctness of Paced Auditory Serial Addition Task (PASAT)-2.0s (r= 0.552, uncorrected P= 0.009) (Figure 5A), and significant moderate positive correlation between the FA changes in the right cerebellar vermis and the changes in the number of the correctness Standard verbal paired-associate learning test (unrelated pairs){S-PA (unrelated)} (r= 0.561, uncorrected P= 0.008) (Figure 5B).DISCUSSION
The results suggest that the combined CCT induced neuroplastic microstructural and functional brain changes detectable by the DSI indices and rsFC. Of these results, the observation of decreased GFA in the right inferior parietal lobule upon CCT may suggest that selective elimination of synapses underlies use-dependent plasticity1. Synaptic pruning for brain maturation progresses rapidly until the late twenties2 and significant negative correlations between IQ and dendrite density or arborization have been reported in healthy young to middle-aged individuals3. The right inferior parietal lobule is known to be a core region in the frontoparietal network. It is involved in motor learning, execution, inhibition, cognition of time and space, reasoning, working memory, and spatial attention4,5, and the right cerebellar vermis is known to involve in language processing6. The right inferior parietal lobule and its neural connections as well as the right cerebellar vermis may play in modulating cognitive functions such as provoking the far-transfer effect.CONCLUSIONS
The effect of cognitive training on neurocognitive performance and its relation to the brain microstructure and function was evaluated. The right inferior parietal lobule and its neural connections and the right cerebellar vermis are thought to play a role.Acknowledgements
This study was supported by (i) the Global Institution for Collaborative Research and Education, Hokkaido University, Japan and (ii) the Grant-in-Aid for scientific research by the Japan Society for Promotion of Science (19K11317).References
- Takeuchi, H., Taki, Y., Sassa, Y., Hashizume, H., Sekiguchi, A., Fukushima, A., Kawashima, R. (2011). Working memory training using mental calculation impacts regional gray matter of the frontal and parietal regions. PLoS One 6, e23175.
- Tang, G., Gudsnuk, K., Kuo, S.H., Cotrina, M.L., Rosoklija, G., Sosunov, A., Sonders, M.S., Kanter, E., Castagna, C., Yamamoto, A., Yue, Z,, Arancio, O., Peterson, B.S., Champagne, F., Dwork, A.J., Goldman, J,, Sulzer, D. (2014). Loss of mTOR-dependent macroautophagy causes autistic-like synaptic pruning deficits. Neuron. 83,1131-1143.
- Genç, E., Fraenz, C., Schlüter, C., Friedrich, P., Hossiep, R., Voelkle, M.C., Ling, J.M., Güntürkün, O., Jung, R.E. (2018). Diffusion markers of dendritic density and arborization in gray matter predict differences in intelligence. Nat Commun. 9, 1905.
- Singh-Curry, V., Husain, M. (2009). The functional role of the inferior parietal lobule in the dorsal and ventral stream dichotomy. Neuropsychologia. 47, 1434-1448.
- Humphreys, G.F., Lambon Ralph, M.A. (2015). Fusion and Fission of Cognitive Functions in the Human Parietal Cortex. Cereb. Cortex. 25, 3547-3560.
- Lesage, E., Hansen, P.C., Miall, R.C. (2017). Right lateral cerebellum represents linguistic predictability. J. Neurosci. 37, 6231-6241.
Figures
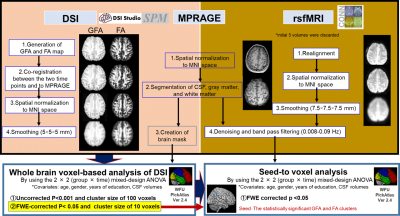
Figure 1. Overview of image processing and statistical analysis.
Image processing of DSI, MPRAGE, and rsfMRI data. The preprocessed GFA and FA were analyzed voxel-by-voxel using the 2 × 2 mixed-design ANOVA to identify brain diffusion changes associated with cognitive training. The statistically significant GFA and FA clusters detected by mixed-design ANOVA are used as seeds to identify rsFC with any other brain region by seed-to voxel analysis. family-wise error (FWE)-corrected cluster-level P< 0.05 was considered statistically significant.
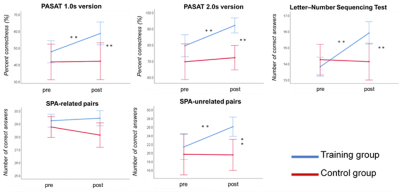
Figure 2. Neuropsychological test performance before and after CCT in the training group.
Significant improvements are observed in the tests that evaluate the far-transfer effects. **p < 0.001. Error bars indicate the standard error. pre=pre-training assessment, post=post-training assessment, PASAT 1.0s = Paced auditory serial addition test (1.0 second paced presentation), PASAT-2.0s = Paced auditory serial addition test (2.0 second paced presentation), SPA= Standard verbal paired-associate learning test.
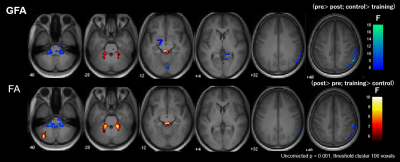
Figure 3. Significant group-by-time interaction observed in GFA and FA.
Clusters with significant interaction in the 2 × 2 mixed-design ANOVA (uncorrected P< 0.001, cluster threshold= 100 voxels) of GFA (upper low) and FA (lower row). The clusters are overlaid on the MPRAGE images. The look-up table indicates F-values for group × time interaction. Cool color indicates post-training assessment < pre-training assessment, training < control, and hot color indicates pre-training assessment < post-training assessment, control< training.
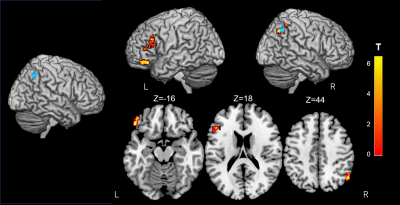
Figure 4. Alterations of resting-state functional connectivity in significant GFA Clusters.
The brain voxels which are functionally connected to the right inferior parietal lobule cluster (Blue) reveal a significant interaction in GFA using a 2 × 2 mixed-design ANOVA. The rsFC between this region and the left frontal pole, the inferior frontal gyrus and the right lateral occipital cortex, increases upon cognitive training (FWE-corrected P< 0.05). Significant clusters are shown on ch2better.nii template using MRIcron. The look-up table indicates the T-value.
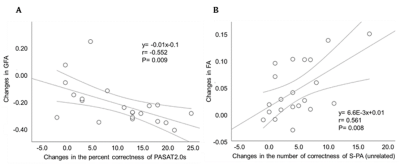
Figure 5. Relationships between the changes of GFA, FA and neuropsychological test performance.
Scatterplots showing significant moderate negative and positive correlations of the change in neuropsychological tests with DSI indices. (A) The relationship between changes in the GFA clusters in the right inferior parietal lobule and the changes in the percent correctness of PASAT2.0s. (B) Correlation between the changes in the right cerebellar vermis and the changes in the number of the correctness of S-PA (unrelated).