2023
Super-resolution and CNN denoising to improve the accuracy of small brainstem structure characterization with in vivo diffusion MRI1Radiology, NYU School of Medicine, New York, NY, United States, 2Neurology, NYU Langone, New York, NY, United States
Synopsis
Diffusion MRI should be sensitive to early pathology or functional re-organization changes for small internal brainstem structures associated with ischemia, multiple sclerosis or neurodegeneration. Application of diffusion MRI to brainstem studies is challenged by limited spatial resolution, image distortion from skull base artifacts and bias introduced if diffusion contrast is also used for structure segmentation. We describe and evaluate a novel combination of Fast Gray Matter Acquisition T1 Inversion Recovery (FGATIR) denoising with deep learning, multi-modal nonlinear image co-registration and super-resolution techniques to improve the accuracy of small internal brainstem structure segmentation on advanced diffusion MRI data.
Introduction
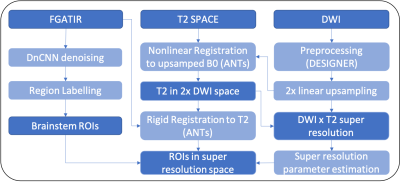
The brainstem is a complex configuration of small nuclei and pathways essential for life, yet it remains challenging to confidently spatially localize brainstem structures using in vivo MRI. Diffusion-weighted MRI may be highly sensitive to early pathologic changes to these structures in patients, but cannot also be used for structure segmentation without bias. There are no consensus, validated atlas-based segmentations of small internal brainstem structures. The 3D Fast Gray Matter Acquisition T1 Inversion Recovery (FGATIR) can provide direct contrast resolution of internal brainstem stuctures1, but we observed that image co-registration and propagation of brainstem structure segmentations onto diffusion MRI data were confounded by echoplanar image distortion in the skull-base and partial volume effects.We developed a new multi-modal pipeline that uses deep learning to denoise FGATIR images, nonlinear registrations with 3D T2 space to correct image distortion, and super-resolution on diffusion-weighted data to reduced partial volume effects in brainstem structures. We compared performance of this pipeline to conventional region-of-interest propagation in a large cohort of healthy control subjects.
Materials and Methods
After informed consent, 20 healthy volunteers (12 female, 56.9 ± 14.1 years) underwent non-contrast MRI protocol using Siemens Magnetom Prisma 3T MRI. The protocol included axial diffusion sequence b = 0 - 2000 s/mm2 along 84 directions (TR/TE: 3500/70 ms, 1.7 x 1.7 x 3.0 mm3, matrix 130 x 130, 54 slices), 1-mm isotropic FGATIR (TR/TE/TI = 3000/1.87/410ms, 256 x 256 matrix, 144 sagittal slices) and 1-mm isotropic T2 SPACE (TR/TE = 3200/422ms; 232 x 256 matrix, 176 sagittal slices). The data then underwent the novel processing pipeline (Figure 1).Diffusion images were processed with DESIGNER3 including denoising, correction for Gibbs effects, Rician bias, eddy currents and EPI distortion. Then, diffusion data was up-sampled 2-fold using linear interpolation, then used as a reference for diffeomorphic registration of the T2 space images using ANTs4. Super-resolution with a self-similarity approach5 was used to recompute the interpolation weights for up-sampled diffusion data, with weights now based on combining a sigma filter in the T2 image with a nonlocal means filter in the interpolated diffusion data. This effectively changes diffusion voxel size from 8 to 1 mm3. Higher weights were given to voxels with similar intensity in the T2-weighted image and with similar local context in the diffusion image. Diffusion kurtosis tensor estimation was performed using a weighted linear least squares fit6 after diffusion interpolation weights were recomputed.
FGATIR images were denoised using modified DnCNN2 architecture. A 20-layer residual discriminative network was trained in 2D using both MRI and non-MRI training images to reduce overfitting bias. Gaussian noise (SNR=1-100) was added for 400 training datasets, along with a validation dataset consisting of 40 images for regularization. FGATIR data was processed by running raw data through the forward model, omitting bias terms, then subtracting the computed noise map. A neuroradiologist with 10 years’ experience labeled 8 brainstem structures with clear boundaries on the denoised FGATIR data – the corticospinal tract in the internal capsule, cerebral peduncle and basis pons (CST, CP & PON, respectively), corticoreticular tract in the internal capsule (CRT), medial longitudinal fasciculus (MLF), red nucleus (RED), pontine and medullary reticular formations (MRF & PRF, respectively). The denoised FGATIR image then underwent rigid body registration to T2 SPACE, such that brainstem labels could be propagated onto the reconstructed diffusion images through the prior computed nonlinear warp.
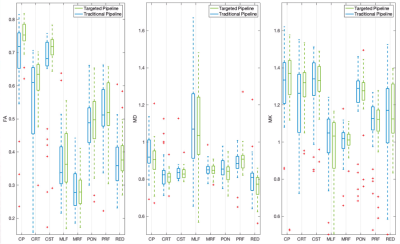
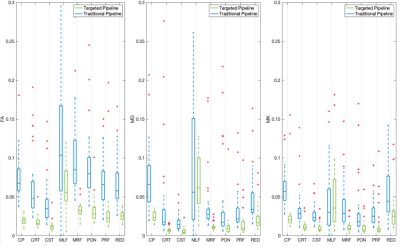
For the 20 subjects, the novel pipeline was compared to conventional ROI propagation where labels were transferred directly to the preprocessed diffusion data through rigid body alignment to the FGATIR. To evaluate the consequence of super resolution on partial volume effects in the diffusion data for each of the 8 brainstem structures, we then compared 1) mean diffusivity, fractional anisotropy and mean kurtosis, and 2) the normalized coefficients of variation for these parameters using the two pipelines.
Results
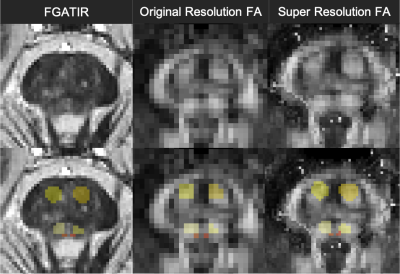
Figure 2 illustrates qualitative improvement for brainstem structure region-of-interest registration fidelity in the diffusion MRI data for the novel super-resolution pipeline compared to the conventional approach. For brainstem structures composed of bundled myelinated white matter and predicted high structural anisotropy (CP, CRT, CST & MLF), the novel pipeline increased FA 1-5% and reduced MD ~2% (Figure 3). This may reflect reduced partial volume effects with adjacent CSF or adjacent brainstem structures with low anisotropy. The coefficients of variation for the MRI parameters also decreased 2-5% for the 8 brainstem structures using the new pipeline (Figure 4).Discussion
This novel label propagation pipeline appears to increase the qualitative spatial accuracy of brainstem segmentation and improve quantitative diffusion precision for brainstem structures segmentation using direct FGATIR image contrast compared to a conventional registration pipeline. The fidelity of brainstem structure segmentation may be further assessed with blinded expert ordinal assessment of registration quality. Higher-resolution diffusion acquisitions, different source images for direct brainstem structure segmentation and further incorporation of deep learning techniques also may improve the results further.Conclusion
The lack of accurate segmentation of small internal brainstem structures reduces our sensitivity to early pathology and potential functional reorganization only evident in postmortem studies of patients. This novel processing pipeline may improve our ability to detect these changes in vivo using diffusion MRI.Acknowledgements
This work was supported by the NIH under awards number R01NS088040 (NINDS), R01EB027075 (NIBIB), R01NS110696 (NINDS), and by the Center of Advanced Imaging Innovation and Research (CAI$$$^2$$$R, www.cai2r.net), a NIBIB Biomedical Technology Resource Center: P41 EB017183.
References
1. Shepherd, T. M., Ades-Aron, B., Bruno, M., Schambra, H. M., & Hoch, M. J. (2020). Direct In Vivo MRI Discrimination of Brain Stem Nuclei and Pathways. American Journal of Neuroradiology, 41(5), 777-784.
2. Zhang, K., Zuo, W., Chen, Y., Meng, D., & Zhang, L. (2017). Beyond a gaussian denoiser: Residual learning of deep cnn for image denoising. IEEE Transactions on Image Processing, 26(7), 3142-3155.
3. Ades-Aron, B., Veraart, J., Kochunov, P., McGuire, S., Sherman, P., Kellner, E., ... & Fieremans, E. (2018). Evaluation of the accuracy and precision of the diffusion parameter EStImation with Gibbs and NoisE removal pipeline. NeuroImage, 183, 532-543.
4. Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011 Feb 1;54(3):2033-44. doi: 10.1016/j.neuroimage.2010.09.025.
5. Lee, H. H., Lin, Y. C., Lemberskiy, G., Ades-aron, B., Baete, S., Boada, F. E., Fieremans, E., Novikov, D. S. (2019). SUper-REsolution TRACTography (SURE-TRACT) pipeline using self-similarity between diffusional and anatomical images. ISMRM 27th Annual Meeting, Montreal, Canada. (27), 0167.
6. Veraart J, Sijbers J, Sunaert S, Leemans A, Jeurissen B. Weighted linear least squares estimation of diffusion MRI parameters: strengths, limitations, and pitfalls. Neuroimage. 2013 Nov 1;81:335-346. doi: 10.1016/j.neuroimage.2013.05.028. Epub 2013 May 16. PMID: 23684865.
Figures