2002
The impact of Marchencko-Pasteur principal component analysis denoising on high-resolution MR spectroscopic imaging in the rat brain at 9.4T.1CIBM Center for Biomedical Imaging, Lausanne, Switzerland, 2Animal Imaging and Technology, EPFL, Lausanne, Switzerland, 3Laboratory of Functional and Metabolic Imaging, EPFL, Lausanne, Switzerland, 4Geneva School of Health Sciences, HES-SO University of Applied Sciences and Arts Western Switzerland, Geneva, Switzerland, 5Department of Radiology, Lausanne University Hospital (CHUV) and University of Lausanne (UNIL), Lausanne, Switzerland
Synopsis
MRSI is a powerful tool for the non-invasive simultaneous mapping of metabolic profiles at multiple spatial positions. This method is highly challenging due to low concentration of metabolites, long measurement times, low SNR, hardware limitations and need for advanced pulse sequences. Denoising based on singular value decomposition has been previously used, but determination of the appropriate thresholds that separate the noise from the signal is problematic leading to possible loss of spatial resolution. Aim of the present study was to implement an improved denoising technique (Marchenko-Pastur principal component analysis) on high resolution MRSI data acquired at 9.4T in the rat-brain.
Introduction
MRSI is a powerful tool for the non-invasive simultaneous mapping of metabolic profiles at multiple spatial positions, and offers an unbiased characterization of the regional differences in the entire brain at a given time point. MRSI is highly challenging due to the low concentration of metabolites, long measurement times, low signal-to-noise ratio (SNR), hardware limitations (B0 and gradient strength, RF coils, B0 inhomogeneities) and the requirement for advanced pulse sequences that often need to be developed in-house. Once data are acquired, there is still a need to develop processing methods, perform quality assessment of a huge number of spectra and estimate the precision and reliability of derived metabolite maps.The need for high spatial resolution combined with the low concentration of metabolites inherently lead to low SNR. Therefore, post-processing methods that aim at minimizing the noise in the MRS signals are needed. Few denoising schemes have been proposed, but surprisingly none was fully adopted by the MRS community1–4. Denoising based on singular value decomposition has been previously used, but the determination of the appropriate thresholds that separate the noise from the signal is problematic leading to possible loss of spatial resolution or the elimination of spectral features that are present in only a small fraction of the voxels.
The aim of the present study was to implement an improved denoising technique on high resolution MRSI data acquired at 9.4T in the rat brain. This technique is also based on principal component analysis (PCA), additionally exploiting the fact that noise eigenvalues follow the universal Marchenko-Pastur (MP) distribution, a result of the random matrix theory. It has already shown great performance on diffusion MRI5, functional MRI6 and DW-MRS7 data . The performance of denoising improves with the number of measurements, which makes the MRSI acquisitions suitable as they contain a large number of spectra (i.e high redundancy).
Methods
The experiments were performed in rat brain on a 9.4T horizontal magnet (Varian Magnex/Scientific). The MRSI data were acquired using an ultra-short echo time SPECIAL spectroscopic imaging sequence (TR=1000/2.8ms)8,9. A matrix size of 32x32 nominal voxels with a field of view (FOV) of 24x24mm2 was acquired, leading to a nominal voxel size of 0.75x0.75x2mm3. First and second order shims were applied using FASTMAP in a voxel of 6x9x2mm3 centered in the rat brain.A sub-matrix of 8x15 voxels centered on the region of interest was selected, resulting in 120 FIDs used in further analysis. The complex-valued FIDs were split into real and imaginary parts and organized into a matrix X, where the first dimension contained the time-domain sampling (1024 points) and the second dimension all the spectra from the selected sub-matrix (240 real+imaginary). Matrix X was denoised using the MP-PCA approach5. Raw and denoised spectra were preprocessed in MATLAB script and quantified with LCModel using an appropriate basis set.
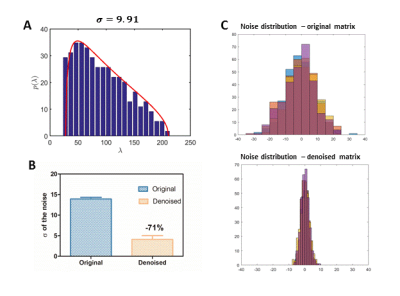
The effect of denoising was evaluated by comparing standard deviations (SD) of the noise before and after denoising on a selected matrix and assessing the SNR of the spectra as well as the Cramer-Rao bounds (CRB) of the metabolite concentrations. The noise SD level was calculated on the last 300 points of the FID before and after denoising.
Results and discussion
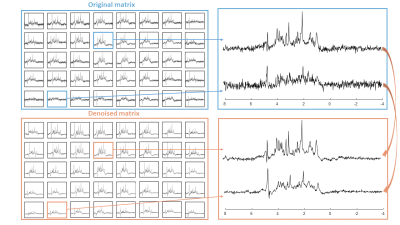
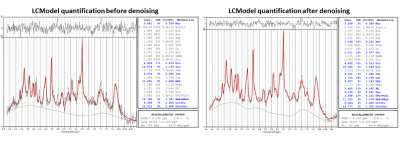
The denoising resulted in accurate fits to the MP distribution (Figure 1A). Figure 1B shows a clear reduction of the noise SD after denoising (-71%). A marked effect of denoising on the spectra in each nominal voxel is visible in Figure 2.The SNR estimated from LCModel quantification increased without any impact on the linewidth or other features of the spectra. The SNR (calculated by LCModel) in the region of interest for quantification was in range of SNR=3 – 10 before denoising and increased to SNR=8 - 20 after denoising. Note that LCModel SNR depends not only on the SD of residuals, but also on the baseline estimate. CRBs were significantly reduced (e.g. from 21% to 9% for Taurine (Tau) and from 33% to 13% for total-Choline (tCho) – Figure 3.), especially for spectra located on the edges of the region of interest where the SNR is particularly low, which makes reliable quantification critical.
Conclusion
MP-PCA denoising significantly improved spectral SNR and metabolite quantification, particularly for spectra located at the edges of the volume of interest (VOI), which led to a better brain coverage. To date there is a growing need for methods that enable the non-invasive imaging of brain metabolism in vivo at a very high spatial resolution, zooming in on specific brain structures. These results are promising and this method offers enormous potential towards novel and fast MRSI developments. Further studies will be performed to evaluate the performance of denoising for fast MRSI acquisitions and to find the optimum between the number of measurements (i.e. spatial resolution, measurement time) and final spectral quality.Acknowledgements
We acknowledge access to the facilities and expertise of the CIBM Center for Biomedical Imaging, a Swiss research center of excellence founded and supported by Lausanne University Hospital (CHUV), University of Lausanne (UNIL), Ecole polytechnique fédérale de Lausanne (EPFL), University of Geneva (UNIGE) and Geneva University Hospitals (HUG).References
1. Ebel A, Dreher W, Leibfritz D. Effects of zero-filling and apodization on spectral integrals in discrete Fourier-transform spectroscopy of noisy data. J Magn Reson. 2006;182:330-338. doi:10.1016/j.jmr.2006.06.026
2. Brender JR, Kishimoto S, Merkle H, et al. Dynamic Imaging of Glucose and Lactate Metabolism by 13 C-MRS without Hyperpolarization. Sci Rep. 2019;9:3410. doi:10.1038/s41598-019-38981-1
3. Ahmed OA. New denoising scheme for magnetic resonance spectroscopy signals. IEEE Trans Med Imaging. 2005;24:809-816. doi:10.1109/TMI.2004.828350
4. Goryawala M, Sullivan M, Maudsley AA. Effects of apodization smoothing and denoising on spectral fitting. Magn Reson Imaging. 2020;70:108-114. doi:10.1016/j.mri.2020.04.013
5. Veraart J, Novikov DS, Christiaens D, Ades-aron B, Sijbers J, Fieremans E. Denoising of diffusion MRI using random matrix theory. Neuroimage. Published online 2016. doi:10.1016/j.neuroimage.2016.08.016
6. Ades-aron B, Lemberskiy MSG, Veraart J, Golfinos J. Improved Task-based Functional MRI Language Mapping in Patients with Brain Tumors through Marchenko-Pastur Principal Component Analysis Denoising. 2020;(7).
7. Jelescu IO, Veraart J, Cudalbu C. MP-PCA denoising dramatically improves SNR in large-sized MRS data : an illustration in diffusion-weighted MRS. ISMRM 2019.
8. Mlynárik V, Kohler I, Gambarota G, Vaslin A, Clarke PGH, Gruetter R. Quantitative proton spectroscopic imaging of the neurochemical profile in rat brain with microliter resolution at ultra-short echo times. Magn Reson Med. 2008;59(1):52-58. doi:10.1002/mrm.21447
9. Cudalbu C, Mlynárik V, Lanz B, Frenkel H, Costers N, Gruetter R. Imaging glutamine synthesis rates in the hyperammonemic rat brain. In: ISMRM. ; 2010:3324.
Figures