1999
Highly accelerated variable-density MultiNet CAIPIRINHA for 1H MRSI and augmented MRSI neural network training
Kimberly Chan1 and Anke Henning1,2
1The University of Texas Southwestern, Dallas, TX, United States, 2Max Planck Institute for Biological Cybernetics, Tübingen, Germany
1The University of Texas Southwestern, Dallas, TX, United States, 2Max Planck Institute for Biological Cybernetics, Tübingen, Germany
Synopsis
It has previously been shown that neural networks combined with variable k-space undersampling (MultiNet GRAPPA) is superior to a conventional GRAPPA reconstruction and is feasible at 7T. Here, MultiNet reconstruction of several new CAIPIRINHA-based variable-density k-space undersampling schemes is investigated. A new approach to train the neural networks (NN) by augmenting the MRSI data with the non-water suppressed (NWS) data to provide additional self-calibration training data is also introduced and evaluated. In this study, both are shown here to reduce lipid artifacts and improve metabolic maps at high acceleration factors relative to those previously proposed for MultiNet GRAPPA.
Purpose
GRAPPA k-space undersampling and reconstruction techniques have been applied to decrease the long MR spectroscopic imaging (MRSI) acquisition times (1-3). We have recently shown that a neural network-based GRAPPA reconstruction combined with variable-density k-space undersampling (MultiNet GRAPPA) is superior to conventional GRAPPA reconstruction and is feasible at 7T (3,4,5). Here, MultiNet reconstruction of several new CAIPIRINHA-based variable-density k-space undersampling schemes is investigated. We also present a new approach to train the neural networks (NN) by augmenting the MRSI data with the non-water suppressed (NWS) data to provide additional training data. Both are shown here to reduce lipid artifacts and improve metabolic maps at high acceleration factors ranging from 5x to 9x relative to those previously proposed for MultiNet GRAPPA (3,4).Methods
Fully sampled 2D 1H FID-MRSI data were acquired in 6 healthy adults (2 male, 4 female, age: 27±2.1 years) on a Philips Achieva 7T scanner with a Nova Medical 32-channel head coil after informed signed consent. Data were acquired with a flip angle=32°, TR=300 ms, TE=1.02 ms, spectral bandwidth=4000-Hz, and 512 points for a total acquisition time of 16 minutes. Other parameters included a field-of-view of 200x200 mm2 in a slice located at the level of the corpus callosum, a slice thickness of 10-mm and a 64x64 phase encoding matrix. NWS data were acquired in the same location with the same parameters without water suppression. An anatomical image with a 256x256 resolution was acquired with a T1-FFE sequence at the same position with the same dimensions as the MRSI data and a flip angle=80°, TR=37 ms, and TE=4 ms.The elliptically-sampled data was retrospectively undersampled with the schemes shown in Figure 1a for acceleration rates of 5x, 6x, and 9x for effective scan times of 3.3, 2.7, and 1.8 minutes respectively. NNs were trained using the MRSI self-calibration data or the augmented MRSI training data as well as 16 semi-synthetic anatomical averages (5). A schematic of the approach to combine the NWS with the MRS data to create an augmented MRSI training data set is shown in Figure 1b. The NWS data was retrospectively sampled by the original 6x mask while the MRSI data was retroactively sampled by a higher acceleration. A HLSVD water filter is applied to the NWS dataset to largely remove the water signal in favor of the lipid signal to allow for the NN to better learn the lipid behavior across k-space. The two data sets are then combined with preference for the MRS data which forms an augmented MRSI set for MultiNet training of the NN.
For each reconstruction, 4 NN were trained to fill the missing points. L1 regularization for retrospective lipid removal was also applied (6). Errors in metabolite map reconstructions were evaluated by calculating the voxel-by-voxel difference between the metabolite maps from the accelerated data and the metabolite maps from the fully sampled data and normalizing the value by the maps from the fully-sampled data. The lipid root-mean-square (RMS) was evaluated and SNR was calculated as the ratio between the NAA peak over the noise root-mean-square.
Results
Figure 2 shows representative spectra (a) and lipid maps (b) in one subject. Qualitatively, the spectra are similar to each other, however, the spectra from the variable-density CAIPIRINHA schemes have lower lipid. The variable-density CAIPIRINHA schemes show reduced lipid artifacts in the brain relative to that from the original 6x acceleration scheme. In figure 3, the median lipid RMS values are lower for the variable-density CAIPIRINHA schemes than for the original 6x-acceleration scheme with p-values < 0.03 while the SNR stays the same. The lipid RMS values are significantly lower for the 9x-accelerated data trained with the augmented MRSI dataset than that trained with just the MRSI. The variable-density CAIPIRINHA schemes and augmented MRSI training set also result in improved metabolite maps with reduced errors relative to the original 6x-acceleration scheme and original 9x-accelerated data reconstruction, respectively, as shown in Figure 5. This can be seen visually in the glutamate+glutamine (Glx) over total creatine (tCr) maps shown in Figure 5 where the maps taken from the variable-density CAIPIRINHA schemes are most similar to Glx/tCr map from the fully-sampled data set.Discussion
The variable-density CAIPIRINHA-based k-space undersampling schemes are shown to reduce lipid artifacts relative to the original GRAPPA-based undersampling schemes when reconstructed with MultiNet. This increased the similarity of the resulting metabolic maps to those from the fully-sampled data set. This reduction in lipid RMS results from the way the 2nd most central region of the k-space is sampled which pushes the lipid aliasing artifacts to the edges of the field-of-view (7). In the 5x-accelerated scheme, this region also provides enough self-calibration points across a large enough region in k-space to capture any non-linearities at the edges of k-space for training the 2-voxel NN (8). In the 6x-accelerated schemes, a second region between the 2nd most central points and the outermost points is needed to provide an extended region to train the 2-voxel NN. In the 9x-accelerated scheme, the water-removed NWS data provided additional self-calibration k-space points towards the edges of k-space which better trained the 2-voxel NN and improved the fidelity of the metabolic maps.Acknowledgements
This work was funded by the Cancer Prevention and Research Institute of Texas (CPRIT) (Grant Number: RR180056) and European Union (Horizon2020, CDS-QUAMRI, Grant Number: 634541).References
1. Hangel et al., NMR in Biomed 20152. Moser et al., Magn. Reson. Med 2019
3. Nassirpour et al. NeuroImage 2018
4. Nassirpour et al. ISMRM 2018
5. Chan et al. ISMRM 2020
6. Bilgic et al. Magn. Reson. Med 2013
7. Breuer et al. Magn. Reson. Med 2006
8. Chang et al. NeuroImage 2012
Figures
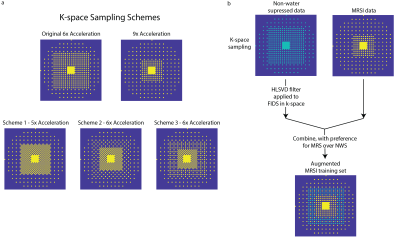
1. (a) K-space undersampling schemes for the originally proposed 6x and 9x-acceleration (first row) as well as the newly proposed variable-density CAIPIRINHA schemes for 5x and 6x acceleration. (b) Schematic for augmenting the MRSI data with water-removed NWS data to provide more k-space points as well as a larger k-space region for training the neural networks based off the lipid behavior.
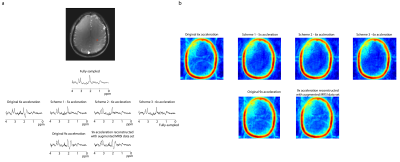
2. (a) Representative spectra in a voxel taken from one subject across different k-space sampling schemes. While the spectra are qualitatively similar to one other and to the spectrum taken from the fully-sampled dataset, the spectra from the variable-density CAIPIRINHA sampling schemes have lower lipid peaks. (b) Lipid maps taken from one subject. The variable-density CAIPIRINHA schemes show reduced lipid artifacts in the brain relative to that from the original 6x acceleration scheme.
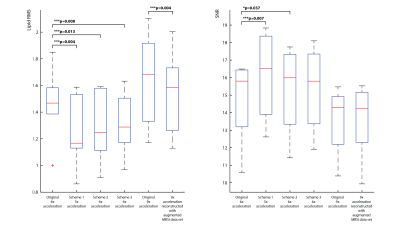
3. Boxplots of the lipid RMS values and SNR values across the slice and across subjects. Median lipid RMS values are lower for the variable-density CAIPIRINHA schemes than for the original 6x-acceleration scheme. The SNR across subjects, however, stays the same.
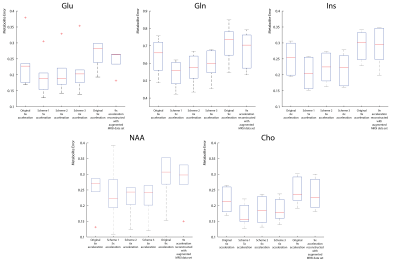
4. Errors in the reconstructed metabolite maps across all 6 subjects for N-acetyl aspartate (NAA), myo-Inositol (mI), choline (Cho), glutamate (Glu) and glutamine (Gln). For all metabolic maps, the variable-density CAIPIRINHA maps show reduced error relative to the original 6x-acceleration scheme while the 9x-accelerated dataset reconstructed with the augmented MRSI dataset also shows reduced error relative to the original reconstruction of the 9x-accelerated data.
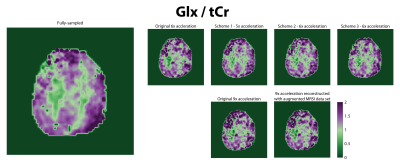
5. Glutamate + glutamine (Glx) maps over total creatine (tCr) in one subject across different sampling schemes and reconstruction methods. Of the maps featured, the Glx/tCr maps from the variable-density CAIPIRINHA schemes are most similar to the Glx/tCr map from the fully-sampled dataset.