1992
Improved prospective frequency correction for macromolecule-suppressed GABA editing with metabolite cycling at 3T1The University of Texas Southwestern, Dallas, TX, United States, 2MR Clinical Science, Philips Health Systems, Horgen, Switzerland, 3Russell H. Morgan Department of Radiology and Radiological Science, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 4. M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States, 5UBC MRI Research Centre, University of British Columbia, Vancouver, BC, Canada, 6Max Planck Institute for Biological Cybernetics, Tübingen, Germany
Synopsis
Macromolecule-suppressed GABA-editing with symmetrical suppression is often preferred over conventional GABA-editing due to its greater specificity. However, this pulse sequence is more sensitive to magnetic field instabilities than conventional GABA-editing. This leads to macromolecule contamination in the edited GABA signal. Here, we combine metabolite cycling with J-difference (MC-MEGA) editing to allow for prospective volume-localized frequency correction at each repetition time without the acquisition of additional water reference transients. We show here that prospective MC-MEGA reduces B0 field instability relative to intermittent prospective frequency correction with water suppressed (WS) MEGA and reduces macromolecule contamination and subtraction artifacts.
Purpose
GABA editing with symmetrical suppression is capable of isolating GABA from macromolecules (MM-suppressed GABA-editing) and correlates more strongly with behavior metrics (1) and tissue distribution differences (2) than conventional GABA-editing. This technique, however, is more sensitive to B0 field instabilities caused by motion or gradient-induced heating or cooling. Here, metabolite cycling is combined with J-difference editing (MC-MEGA) to allow for continuous prospective frequency correction without additional acquisitions and implemented for MM-suppressed GABA-editing. Its performance is compared to water-suppressed MEGA-PRESS (WS-MEGA) editing with intermittent prospective frequency correction (4) in phantoms and in the occipital lobe (OCC) the medial prefrontal cortex (mPFC) regions of the human brain. This implementation represents the first use of MC for prospective frequency correction which was previously only implemented for retrospective frequency correction. This technique also contrasts with the previously proposed WS-MEGA which required additional water reference acquisitions interleaved with the spectral acquisitions.Methods
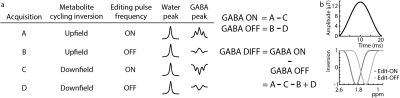
Figure 1a shows a schematic of the MC-MEGA steps and consists of alternate inversion of the upfield and downfield spectrum for each editing step (ON/OFF). Prospective frequency correction was implemented as in reference 4. The acquired FID was Fourier transformed and the frequency offset was calculated and added to the spectrometer center frequency prior to the next transient acquisition. In WS-MEGA, this was done intermittently based on an additionally acquired water reference every 20 water-suppressed transients (3). In MC-MEGA, this was done at every transient without the need of separate water reference acquisitions due to the simultaneous acquisition of metabolite and water data.All experiments were performed on a Philips Achieva 3T scanner with 20-ms editing pulses (bandwidth=62-Hz) (Figure 1b) applied at 1.9 ppm and 1.5 ppm in the edit-ON and edit-OFF acquisitions respectively. Other acquisition parameters included TE=80-ms, 320 transients, and VAPOR water suppression for WS-MEGA (4).
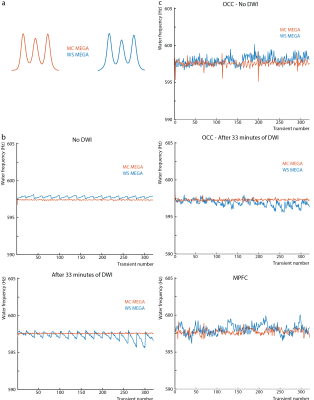
Phantom experiments were performed in a (2.2 cm)3 voxel in a 30 mM GABA phantom with a TR of 2.4s with and without a prior 32-minute diffusion-weighted MRI (DWI) scan. The transmit frequency of the MC pre-inversion pulse was shifted by -170-Hz and 170-Hz for the upfield and downfield RF pulse respectively.
MRS data was acquired in 16 healthy volunteers (9 female, 7 male, age 25.4±2.6 years) in a (3 cm)3 region in the OCC with and without prior 32-minute DWI in 7 and 5 volunteers respectively and in a 2.5x3.5x3 cm3 region in the mPFC in 8 volunteers. The transmit frequency of the metabolite cycling pre-inversion pulse was shifted by -180-Hz and 180-Hz for the upfield and downfield RF pulse respectively. For all WS-MEGA scans, TR=2.4s, while in the MC-MEGA scans, TR=2.5s and TR=3.4s in the mPFC and OCC respectively. The difference in TRs arise from the adjustment made to the nominal TR to account for the extra 1s to perform the frequency update in the mPFC scans but not in the OCC scans.
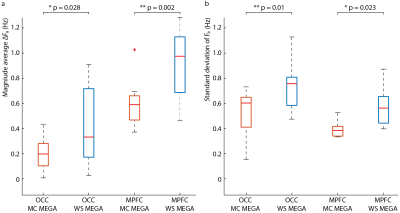
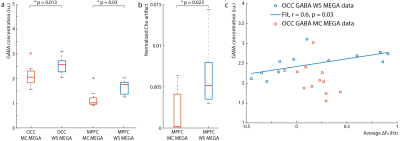
Data were analyzed using in-house software and Gannet (5) with retrospective phase and frequency correction performed in the time domain (6). For the OCC data, the GABA peak was modeled with a Gaussian from 2.82-3.25 ppm. Since the MPFC data contained subtraction artifacts, both the residual choline (Cho) artifact at 3.2-ppm and the GABA/Cr peak at 3.0-ppm were fit simultaneously from 2.75-3.35 ppm with a Lorentzian and Gaussian model, respectively. GABA concentrations were estimated in institutional units (i.u.). The magnitude average difference in the water frequency (ΔF0) from 4.68-ppm and the standard deviation of the water frequency were calculated across transients.
Results
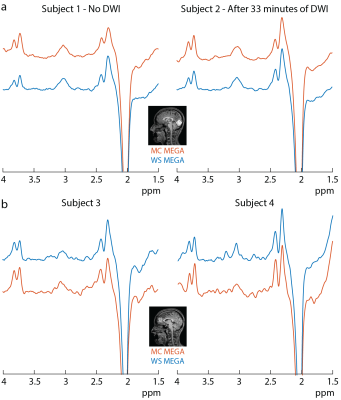
The edited GABA multiplet in the phantom was qualitatively similar between WS-MEGA and MC-MEGA (Figure 2a). In both phantom and in vivo experiments (Figure 2b and 2c) MC-MEGA sequence has a more consistent water frequency over acquisition time. In vivo, WS-MEGA and MC-MEGA spectra in the OCC are qualitatively similar (Figure 3a). In the MPFC, significant Cho subtraction artifacts can be seen in WS-MEGA but not MC-MEGA (Figure 3b). WS-MEGA acquisitions also have a significantly higher magnitude average ΔF0 and water frequency standard deviation than MC-MEGA in the OCC and mPFC (Figure 4a, 4b). In both regions, the GABA concentrations are higher in WS-MEGA than in MC-MEGA with median (interquartile range) values of 2.52 (0.45) i.u. and 2.03 (0.48) i.u. respectively in the OCC and 1.02 (0.26) i.u. and 1.77 (0.48) i.u. respectively in the mPFC likely due to MM contamination (Figure 5a). The WS-MEGA GABA-edited spectra contain significantly higher Cho artifacts than MC-MEGA (Figure 5b). WS-MEGA GABA concentrations in the OCC but not MC-MEGA GABA concentrations demonstrated a moderate correlation with the average ΔF0 (Figure 5c).Discussion
MC-MEGA allows for continuous frequency correction without additional acquisitions and decreases the amount of B0 field instability relative to conventional WS-MEGA with intermittent prospective frequency correction. This allows for more consistent on-resonance editing which reduces MM contamination as evident here from the reduced dependency of the GABA concentrations on frequency offset in the OCC, consistent with reference 4 as well as the reduced GABA levels measured with MC-MEGA. These benefits are unavailable with retrospective frequency correction which can only account for line-broadening, lineshape distortion, and subtraction artifacts (3,6). MC-MEGA also reduces the appearance of subtraction artifacts in the mPFC even after retrospective correction.Acknowledgements
This work was funded by the Cancer Prevention and Research Institute of Texas (CPRIT) (Grant Number: RR180056), European Union (Horizon2020, CDS-QUAMRI, Grant Number: 634541), and the National Institute of Health (Grant Number: P41 EB015909, R01 EB023963 and R01 EB016089)References
1. Mikkelsen et al. Brain Research 2018
2. Moser et al., NeuroImage 2019
3. Edden et al, J. Magn. Reson. Imaging 2016
4. Tkác et al. Magn. Reson. Med. 1999
5. Edden et al. J. Magn. Reson. Imaging 2013
6. Near et al. Magn. Reson. Med. 2015.
Figures