1991
Long-TE mixed flip angle editing of GABA1Russell H. Morgan Department of Radiology and Radiological Science, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 2F. M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States, 3Department of Diagnostic Radiology and Nuclear Medicine, University of Maryland School of Medicine, Baltimore, MD, United States
Synopsis
The aim of this work was to investigate an alternative MRS approach for GABA editing. By performing a range of simulations, we found a long-TE scheme that reduces the influence of co-edited macromolecular signal without lower GABA losses than long-TE MEGA-PRESS. We termed this scheme ‘Mixed flip angle’ (MFA) editing, and validated the scheme with phantom measurements and a proof-of-principle in vivo measurement. Preliminary results show that longer-TE editing of GABA has the potential to increase selectivity by allowing for longer editing pulse duration, avoiding the need for symmetrical suppression of MM signals.
Introduction
Edited MRS of GABA is usually performed using a MEGA editing scheme1 with editing pulses applied at 1.9 ppm during the ON steps and at 7.5 ppm during the OFF steps at a TE of 68 ms2. The duration and thus selectivity of editing pulses is TE-limited, resulting in partial inversion of macromolecular (MM) signals at 1.7 ppm and co-edited MM signals at 3 ppm. This measurement is often referred to as GABA+, where the MM contribution is as high as 50% of the total signal3. Experimental options for addressing co-edited MM include symmetrical editing (1.9/1.5 ppm)4, and pre-inversion to yield GABA-nulled MM spectra. Both these approaches have disadvantages, most notably, sensitivity to motion and SNR efficiency, respectively. Editing at longer-TE has two potential advantages, allowing for more selective editing pulses, and greater relaxation losses of the shorter-T2 MM signal. The aim of this work was to investigate alternative approaches for GABA editing using a longer TE. After performing a range of simulations in order to investigate the TE- and flip-angle-dependence of the edited signal, we developed a mixed flip angle (MFA) editing experiment performed at TE 110 ms. This scheme was evaluated with further simulations and phantom measurements, and proof-of-principle in vivo data from one subject are presented.Methods
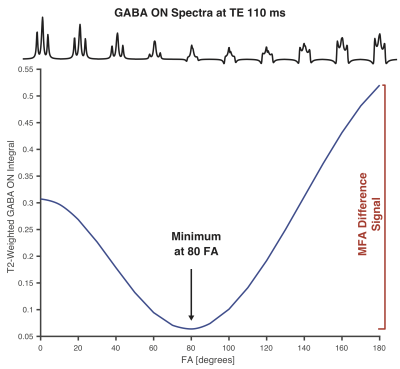
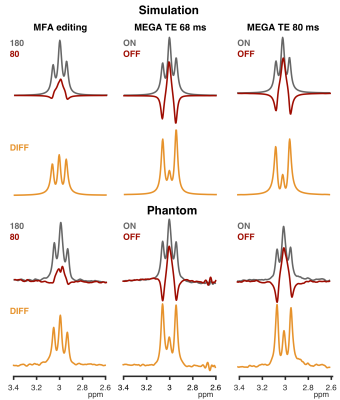
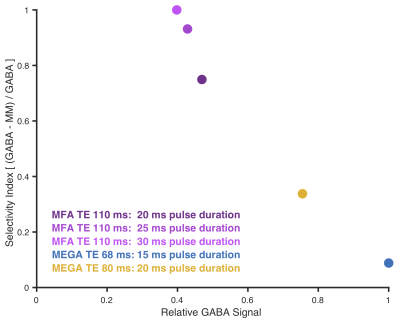
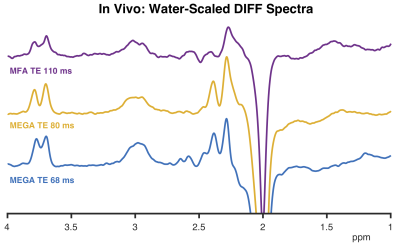
Density-matrix simulations were performed in MATLAB using FID-A5, at a range of TEs and editing pulse flip angles (FA; 0-180º in steps of 10º). All simulations were performed using ideal excitation, experimental refocusing and 20-ms universal editing pulses. It was noted that at longer-TE, there is a GABA signal minimum for FA ~80º (Figure 1), raising the possibility that this might replace the OFF acquisition in a MEGA-PRESS experiment. Thus, instead of having one ON- and one OFF-experiment, the MFA approach utilized two ON editing experiments at 1.9 ppm, but using two different flip angles, 80º and 180º. MM simulations were performed using the lysine spin-system6 to investigate MFA co-editing. A selectivity index (GABA-MM)/GABA was computed for: MFA at TE 110 ms; and MEGA-PRESS at TE 68 and 80 ms, where 1 indicates best selectivity. Finally, all integrals were T2-corrected using T2 88/40 ms for GABA7/MM-lysine8. Experiments were performed on a Philips 3T scanner with a 32-channel head coil, performing three separate acquisitions with the following settings: MEGA-PRESS with TE/TR 68/2000 ms, ON/OFF at 1.9/7.5 ppm, 180º edit FA, 15 ms pulse duration; MEGA-PRESS with TE/TR 80/2000 ms, ON/OFF at 1.9/7.5 ppm, 180º edit FA, 20 ms pulse duration; MFA editing with TE/TR 110/2000 ms, editing pulses at 1.9 ppm, 80/180º edit FA, 20 ms pulse duration. Phantom measurements were performed on a 10 mM GABA phantom: 24x24x24 mm3 voxel; 128 transients. The in vivo measurement was performed on a healthy volunteer, with a 30x30x30 mm3 midline parietal voxel, collecting 320 averages for each acquisition. Both phantom and in vivo data were analyzed using Gannet9. Frequency and phase correction of in vivo data was performed with robust spectral registration10. The in vivo spectra were water-scaled using the water reference signal and relaxation correction.Results
Good agreement between the simulations and the phantom data (Figure 2) indicates that the implementation works as intended. For MEGA-PRESS, increasing the TE from 68 to 80 ms and pulse duration from 15 to 20 ms reduced the co-edited MM signal at 3.0 ppm by 35% at the cost of losing 22% of the GABA signal. The MFA scheme gives 87% less MM signal at the cost of losing 53% of the GABA signal (compared to MEGA-PRESS at TE 68 ms). These numbers are reflected by the simulated selectivity indices plotted in Figure 3. Figure 4 illustrates the resulting water-scaled difference spectra computed for the in vivo measurements.Discussion
This first proof-of-principle investigation illustrates the potential of MFA editing for longer-TE detection of GABA. Given the substantial limitations and poor uptake of currently available approaches to address co-edited MM in GABA measurements, the recent consensus requirements11 to do so necessitates new experimental approaches. The GABA+ experiment is relatively stable but uncertainty over how much co-edited MM signal contaminates the resulting GABA+ measurement (which could be different between subjects or patient populations) limit its scientific utility. Increasing the TE from 68 to 110 ms accommodates editing pulses with longer duration and narrower bandwidth, which improves the editing selection without resorting to symmetrical suppression. Thus, the MM signal will both be more decayed due to the T2-relaxation and less co-edited due to the narrower pulses. Calculating the selectivity index for each acquisition schemes quantifies the trade-off between selectivity and GABA signal efficiency. By increasing the MFA pulse duration to 30 ms, the co-edited MM-signal could be completely removed at the cost of losing about 60% of the GABA signal. This work is preliminary and further measurements are needed in order to see how T2 relaxation and MM inter-subject variability affect in vivo measurements of the GABA signal. Future work also includes testing the sequence in vivo using longer pulse durations, which from simulations show promising results.Conclusion
Mixed flip-angle editing allows more selective detection of GABA than is possible with MEGA-PRESS, avoiding the need for motion-sensitive symmetric suppression of MM.Acknowledgements
This work was supported by NIH grants R01 EB016089, R01 EB023963, R01 MH106564 and P41 EB015909.References
1. Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 1998;11(6):266-272.
2. Mullins PG, McGonigle DJ, O’Gorman RL, et al. Current practice in the use of MEGA-PRESS spectroscopy for the detection of GABA. Neuroimage. 2014;86:43-52.
3. Harris AD, Puts NAJ, Barker PB, Edden RAE. Spectral-editing measurements of GABA in the human brain with and without macromolecule suppression. Magn Reson Med. 2015;74(6):1523-1529.
4. Henry P-G, Dautry C, Hantraye P, Bloch G. Brain GABA Editing without Macromolecule Contamination. Vol 45.; 2001.
5. Simpson R, Devenyi GA, Jezzard P, Hennessy TJ, Near J. Advanced processing and simulation of MRS data using the FID appliance (FID-A)—An open source, MATLAB-based toolkit. Magn Reson Med. 2017;77(1):23-33.
6. Deelchand DK, Marjańska M, Henry P-G, Terpstra M. MEGA-PRESS of GABA+: Influences of acquisition parameters. NMR Biomed. 2019
7. Edden RA, Intrapiromkul J, Zhu H, Cheng Y, Barker PB. Measuring T2 in vivo with J-difference editing: application to GABA at 3 Tesla. J Magn Reson Imaging. 2012;35(1):229-234.
8. Murali-Manohar S, Borbath T, Wright AM, Soher B, Mekle R, Henning A. T(2) relaxation times of macromolecules and metabolites in the human brain at 9.4 T. Magn Reson Med. 2020;84(2):542-558.
9. Edden RAE, Puts NAJ, Harris AD, Barker PB, Evans CJ. Gannet: A Batch-Processing Tool for the Quantitative Analysis of Gamma-Aminobutyric Acid–Edited MR Spectroscopy Spectra. J Magn Reson Imaging. 2014;40(6):1445-1452.
10. Mikkelsen M, Tapper S, Near J, Mostofsky SH, Puts NAJ, Edden RAE. Correcting frequency and phase offsets in MRS data using robust spectral registration. NMR Biomed. 2020;33(10):e4368.
11. Cudalbu C, Behar KL, Bhattacharyya PK, et al. Contribution of macromolecules to brain (1) H MR spectra: Experts’ consensus recommendations. NMR Biomed. November 2020:e4393.
Figures