1990
SLOW: Whole Brain Spectral Editing EPSI Based Technique using Chemical Selective Adiabatic 2π-Refocusing Pulses applied to 2HG and GABA Editing
Guodong Weng1, Claus Kiefer1, Irena Zubak2, and Johannes Slotboom1
1Institute for Diagnostic and Interventional Neuroradiology, Support Center for Advanced Neuroimaging (SCAN), University of Bern, Bern, Switzerland, 2Department of Neurosurgery, Inselspital Bern and University Hospital, Bern, Switzerland
1Institute for Diagnostic and Interventional Neuroradiology, Support Center for Advanced Neuroimaging (SCAN), University of Bern, Bern, Switzerland, 2Department of Neurosurgery, Inselspital Bern and University Hospital, Bern, Switzerland
Synopsis
To overcome the B1+ inhomogeneities and SAR limitation for using MRSI-editing at UHT (≥7T), a new spectral editing scheme was developed within an EPSI sequence for whole brain MRSI. The slice-selective refocusing pulse was replaced by a chemical-selective adiabatic 2π-refocusing pulse pair with varying passbands. The results show an excellent glutamate, 2HG and GABA-editing pattern with in vitro and in vivo measurements. This study has shown the excellent performance of the proposed new spectral editing scheme, and proved that it could be an alternative method for MEGA editing.
INTRODUCTION
Due to better its better SNR, localized spectroscopy is predestined for application at ultra-high file (UHF) (≈7T). In practice, however, many hurdles related to the underlying physics, have to be overcome. Apart from SAR ($$$ \propto $$$B02), the wavelength of the EM-wave (≈11cm at 7T) is shorter than the anatomic structures to be examined which often results in severe interference patterns (inhomogeneities) in the B1+. The B1+-inhomogeneities can partially be overcome by the use of adiabatic RF-pulses 1 or with parallel transmit techniques 2. However, due to practical limitations on the maximum available B1+ and the relatively high SAR of adiabatic spatial selective refocusing pulses, their number should be kept as low as possible. Additionally, their high SAR sets clear limits the practical available RF-bandwidth ($$$ \Delta \omega_{RF} $$$) drastically, which in its turn promotes the chemical shift displacement artifact CSDA 3. However, larger $$$ \Delta \omega_{RF} $$$ is needed in UHF MR-spectroscopy applications since $$$ \Delta \omega_{RF} \propto B_0$$$. Spectral editing for the detection of e.g. 2HG or GABA is a technique that can be combined with volume localization schemes by adding MEGA editing-pulses 4 adding additional SAR to the sequence. Many MEGA implementations use pure amplitude modulated Gaussian shaped p-pulses,5,6 whose editing performance suffers at high field from severe B1+ inhomogeneities in the clinical 1Tx mode. Therefore, an adiabatic MEGA-editing scheme was developed in 1D-semiLASER (Localization by Adiabatic SElective Refocusing) sequence 7. This abstract proposes a new editing scheme completely different than MEGA which we integrated into EPSI 8 allowing for whole brain 2HG or GABA edited EPSI-MRSI. The novel editing method will be further referred to as SLOW, in analogy to MEGA. SLOW consists only of 2 RF-pulses: apart from a slab selective RF-excitation pulse, only one chemical shift selective adiabatic 2π-pulse with varying passbands. The general properties of single shot EPSI-sequence using 2π-pulses are described in a separate abstract. This abstract focuses on the editing properties of variable bandwidth 2π-pulses.METHODS
Figure 1 below shows the adapted EPSI pulse sequence, in which the original slice selective refocusing pulse was replaced by an adiabatic complex secant hyperbolic RF-pulse pair $$$ B_1^+(t) = \Omega_0 \cdot sech(\beta t)^{1+\mu i} $$$ 9 selectively refocusing two different offset frequency ranges mimicking editing “off” and “on” of MEGA-typed editing. In SLOW-editing we refer “editing-off” as “editing full” and “editing-on” as “editing-partial. In analogy to MEGA-editing, the type of editing is further referred to SLOW-editing. In vitro tests were carried out on three spherical phantoms (1.) brain metabolite phantom (GE), (2.) home-build 2hydroxyglutarate (2HG)-phantom (~7.8 mmol/L of 2HG and 18 mmol/L of glycine), and (3.) home-build GABA-phantom (~10 mmol/L of GABA, creatine, and glycine). In vivo test was performed on one healthy volunteer. All measurements were performed on a 7T MAGNETOM Terra MR-scanner (Siemens, Germany) in clinical-mode.In Figure 2a the frequency selective refocusing offset frequency ranges are displayed for the 2HG-edited SLOW-EPSI whole brain MRSI. During the first acquisition the whole spectrum from 1.8 ppm till 4.2 ppm is refocused (denoted by editing-full), whereas the second echo is obtained refocusing the 0.8 ppm till 3.2 ppm range (denoted by editing-partial). Like in MEGA-editing, SLOW-editing also required the two responses to be subtracted.
As a second example of SLOW-editing GABA editing can be similarly be performed by selectively refocusing the 1.65 ppm-4.2 ppm during editing “full” and 2.7 ppm till 4.2 ppm editing “partial” phase.
RESULTS AND DISCUSSION
Figure 2b-d shows the in vitro result of SLOW-editing for different echo times. As expected, the editing effect shows a clear TE-dependence, indicating that the TE=120ms may be the optimal TE for 2HG-detection at 7T.Figure 3 shows the EPSI-SLOW pulse sequence applied on a phantom containing the main brain metabolites, however no GABA. The SLOW-full refocuses the offset range between 1.65-4.2 ppm whereas the SLOW-partial refocuses 2.7-4.2 ppm range. The difference spectrum very nicely shows the editing effect on glutamine/glutamate multiplet at approximately 3.75 ppm.
Figure 4 shows the effect of SLOW-editing on a GABA and glycine and creatine containing phantom.
Figure 5 shows the in vivo results of the SLOW-EPSI editing sequence for various TE in the range [68 – 110 ms]. The displayed the sum volume of 3 voxels being 13 X 11 X 15 mm. The optimal TE for GABA editing was 68 msec, confirming the in vitro results of Figure 4.
CONCLUSION
SLOW-editing has been presented which is an alternative method for spectral editing using MEGA editing based on adiabatic 2π-pulses and was integrated into a 3D-EPSI pulse sequence and tested at 7T. Since this pulse sequence requires only one slab selective excitation pulse and one adiabatic chemical shift selective 2π-pulse having variable passbands for editing the SAR can be kept extremely low. In contrast to MEGA-editing, SLOW-editing uses adiabatic refocusing and is therefore robust toward B1+-inhomogeneities which are inherent at UHF MRI/MRS. Finally, due to the use of the small band chemical shift selective adiabatic 2π-pulse, there is no in-plane CSDA, and a minimal CSDA perpendicular due to slab selective excitation pulse.Acknowledgements
This work was supported by the Swiss National Science Foundation grant number 182569. Additionally, this project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 813120.References
- Slotboom J, Mehlkopf A., Bovée WMM. A single-shot localization pulse sequence suited for coils with inhomogeneous RF fields using adiabatic slice-selective RF pulses. J Magn Reson. 1991 Nov;95(2):396–404.
- Blaimer M, Breuer F, Mueller M, Heidemann RM, Griswold MA, Jakob PM. SMASH, SENSE, PILS, GRAPPA. How to Choose the Optimal Method [Internet]. Vol. 15, Topics in Magnetic Resonance Imaging. Top Magn Reson Imaging; 2004 [cited 2020 Dec 16]. p. 223–36. Available from: https://pubmed.ncbi.nlm.nih.gov/15548953/
- Slotboom J, Mehlkopf AF, Bovee WMMJ. The Effects of Frequency-Selective RF Pulses on J-Coupled Spin-1/2 Systems. J Magn Reson Ser A. 1994 May;108(1):38–50.
- Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 1998 Oct;11(6):266–72.
- Terpstra M, Marjanska M, Henry PG, Tkáč I, Gruetter R. Detection of an antioxidant profile in the human brain in vivo via double editing with MEGA-PRESS. Magn Reson Med [Internet]. 2006 [cited 2020 Dec 16];56(6):1192–9. Available from: https://pubmed.ncbi.nlm.nih.gov/17089366/
- Chan KL, Puts NAJ, Schär M, Barker PB, Edden RAE. HERMES: Hadamard encoding and reconstruction of MEGA-edited spectroscopy. Magn Reson Med [Internet]. 2016 Jul 1 [cited 2020 Dec 16];76(1):11–9. Available from: https://pubmed.ncbi.nlm.nih.gov/27089868/
- Moser P, Hingerl L, Strasser B, Považan M, Hangel G, Andronesi OC, et al. Whole-slice mapping of GABA and GABA + at 7T via adiabatic MEGA-editing, real-time instability correction, and concentric circle readout. Neuroimage. 2019 Jan 1;184:475–89.
- Ebel A, Maudsley AA. Improved spectral quality for 3D MR spectroscopic imaging using a high spatial resolution acquisition strategy. Magn Reson Imaging [Internet]. 2003 [cited 2020 Dec 14];21(2):113–20. Available from: https://pubmed.ncbi.nlm.nih.gov/12670597/
- Conolly S, Nishimura D, Macovski A. A selective adiabatic spin-echo pulse. J Magn Reson. 1989 Jun;83(2):324–34.
Figures
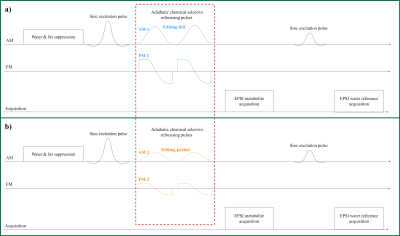
Figure
1:
EPSI-SLOW spectral editing sequence scheme. a) Chemical selectively
refocusing the full range of interested spins. b) Chemical selectively
refocusing the partial range of interested spins.
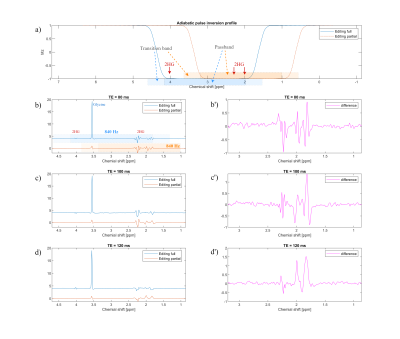
Figure 2: a)
Simulation of the adiabatic pulse (used as inversion pulse in the simulation for
simplicity). The passband and transition band are indicated by blue/orange and
light blue/orange respectively for editing full and editing partial pulses. b)
The editing full/partial pulse bandwidth is 840 Hz but with a different carrier
frequency. TR = 1500 ms, volume of interest (VOI) = 280 X 220 X 100 mm, voxel
size = 4.3 X 11 X 13.8 mm, and total measurement time = 6 mins.
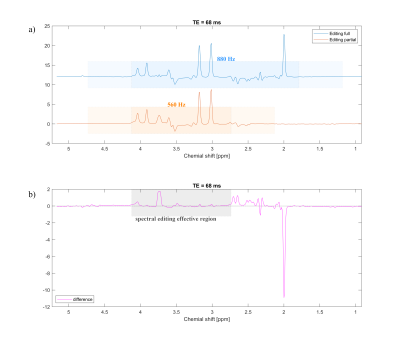
Figure
3:
In vitro measurement on a phantom containing NAA, Glu, Cr, Cho, … (General
Electric phantom). a) The editing full pulse (blue) is 880 Hz BW with a
carrier frequency at 3.15 ppm, while editing partial pulse is 560 Hz BW with a
carrier frequency at 2.6 ppm. b) Difference of editing full/partial
spectral, the gray area indicates the effective spectral editing region. TR =
1500 ms, VOI = 280 X 220 X 100 mm, voxel size = 4.3 X 11 X 13.8 mm, and total
measurement time = 6 mins.
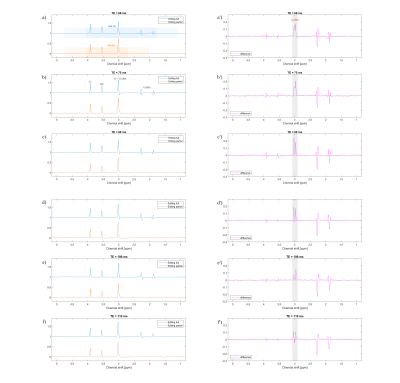
Figure
4:
GABA phantom 1-slice measurement. TR = 1500
ms, VOI = 280 X 220 X 15 mm, voxel size = 4.3 X 11 X 15 mm, and total
measurement time = 1 min.
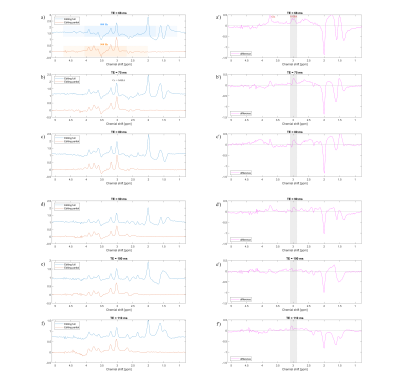
Figure 5: In vivo measurement
(average of 3 voxels). TR = 1500 ms, VOI = 280 X 220 X 100 mm, voxel size = 4.3
X 11 X 13.8 mm, and total measurement time = 6 mins.