1989
Spectral registration for real-time frequency correction of single-voxel GABA-edited MRS data: Proof of concept1Russell H. Morgan Department of Radiology and Radiological Science, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 2F. M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States
Synopsis
Offsets in B0 field frequency have a detrimental impact on the SNR, linewidth, and editing efficiency of edited 1H MRS data. We have previously described an interleaved water referencing (IWR) method for interspersed acquisition of the unsuppressed water signal to correct frequency drift at regular intervals. Here, we add to IWR a spectral registration (SR) approach for updating the center frequency every TR. Proof-of-concept results from phantom and in vivo experiments demonstrate that SR can be successfully combined with IWR for fully prospective correction of frequency offsets across the whole edited MRS scan.
Introduction
B0 frequency drift during 1H MRS experiments will lead to global changes in the observed chemical shifts of metabolites. This offset can be caused by heating/cooling of scanner hardware elements (1) and is particularly prominent following sequences with high gradient duty cycles such as DTI and EPI (2,3). Frequency drift will lead to decreased SNR, increased linewidths, and, in the case of edited MRS, reduced editing efficiency and increased subtraction artifacts (4). Both prospective (5–7) and retrospective (8–10) techniques exist to correct this effect. In this proof-of-concept study, we implement a novel method for real-time frequency correction of GABA-edited MRS data that combines both interleaved water referencing (IWR) (3) and spectral registration (SR) (9).Methods
Real-time spectral registrationIWR integrates voxel-localized unsuppressed water acquisitions into single-voxel water-suppressed MRS acquisitions to both obtain an internal water reference for quantification and to use the observed frequency shift in the water signal to update center frequency (F0). However, the limitation of IWR is that it only updates F0 after each water reference is acquired.
To address this, we introduce SR for shot-to-shot frequency correction across an entire MEGA-PRESS (11) scan. SR has previously been used for retrospective frequency-and-phase correction (9,12,13), but its concept can in theory be applied to work in a prospective manner. The proposed approach uses the acquired FIDs of each water-suppressed acquisition for F0 updates by “co-registering” them to a reference FID. This can be formulated as:
$$\hat{S}_m(\omega)=\left|FFT\left[\frac{S_m(t)}{S_{ref}(t)}\right]\right|$$
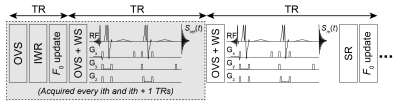
where Sm(t) is the complex FID currently in the scanner’s memory buffer during a given TR, Sref(t) is the complex reference FID, and $$$\hat{S}_m(\omega)$$$ is the estimated “frequency-shift-difference” magnitude spectrum. The frequency update (in Hz) is calculated by determining the frequency difference between the observed residual water signal in $$$\hat{S}_m(\omega)$$$ and the F0 of the spectrometer. A schematic of the IWR + SR prospective frequency correction method for MEGA-PRESS is shown in Figure 1. Note that the IWR component only occurs every ith TR, with Sref(t) being acquired after each IWR reference (i.e., every ith + 1 TR).
Data collection
To assess the efficacy of real-time SR, B0 frequency drift was induced using an EPI scan that preceded a series of single-voxel GABA-edited MEGA-PRESS scans with and without IWR or SR turned on. Phantom and in vivo experiments were performed at 3T on a Philips Ingenia Elition X scanner using a 32-ch phased-array head coil. A cylindrical phantom with 10 mM of GABA was scanned at room temperature using the following protocol:
- MEGA-PRESS without frequency correction (baseline)
- BOLD-weighted EPI
- MEGA-PRESS without frequency correction
- MEGA-PRESS with IWR only
- MEGA-PRESS with IWR + SR
One consenting healthy adult male (32 years old) was also scanned under local IRB approval using the same protocol and acquisition parameters as above, with the exception of VAPOR for water suppression. The voxel was placed medially in the parietal lobe.
As a primary outcome measure, the relative change in center frequency (ΔF0, in Hz) was calculated as the frequency difference between the observed residual water signal in each shot and the nominal water frequency (4.8 ppm in room-temperature phantom, 4.68 ppm in human brain).
Results
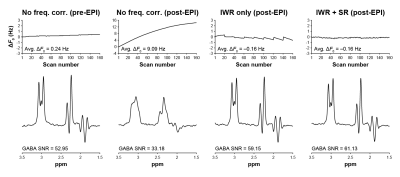
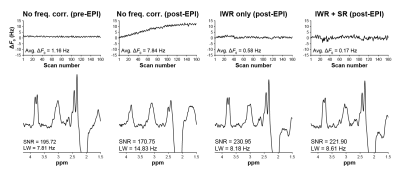
The phantom experimental results are shown in Figure 2. The post-EPI MEGA-PRESS acquisition without frequency correction showed the expected monotonic change in ΔF0 over the duration of the scan. The sawtooth pattern in the IWR-only MEGA-PRESS scan clearly shows the F0 updates occurring when the 8 water references were acquired every 20 TRs. Notably, the use of IWR + SR in the final scan shows a stable B0 frequency without the sawtooth pattern. The scans with IWR only and with IWR + SR both showed the lowest average ΔF0 (both –0.16 Hz) and the highest SNR of the 3.0 ppm edited GABA signal (59.15 and 61.13, respectively).The in vivo experimental results are shown in Figure 3. The frequency offsets show a similar pattern to the phantom experiments. The MEGA-PRESS scan with IWR + SR resulted in the lowest average ΔF0 (0.17 Hz). The SNR and linewidth of the 2.0 ppm NAA signal detected in the edit-OFF spectrum were similar between the IWR-only and IWR + SR scans (SNR: 230.95 and 221.90; linewidth: 8.18 and 8.61 Hz, respectively).
Conclusion
Spectral registration is shown to be a feasible and promising technique for real-time shot-to-shot correction of B0 frequency offsets in edited MRS experiments. The preliminary data presented here will be followed up with more rigorous and extensive tests incorporating real-world experimental challenges to B0 field stability, such as in pediatric and clinical scans.Acknowledgements
This work was supported by NIH grants K99 EB028828, R01 EB016089, R01 EB023963, and P41 EB015909.References
1. Foerster BU, Tomasi D, Caparelli EC. Magnetic field shift due to mechanical vibration in functional magnetic resonance imaging. Magn. Reson. Med. 2005;54:1261–1267 doi: 10.1002/mrm.20695.
2. Harris AD, Glaubitz B, Near J, et al. Impact of frequency drift on gamma-aminobutyric acid-edited MR spectroscopy. Magn. Reson. Med. 2014;72:941–948 doi: 10.1002/mrm.25009.
3. Edden RAE, Oeltzschner G, Harris AD, et al. Prospective frequency correction for macromolecule-suppressed GABA editing at 3T. J. Magn. Reson. Imaging 2016;44:1474–1482 doi: 10.1002/jmri.25304.
4. Evans CJ, Puts NAJ, Robson SE, et al. Subtraction artifacts and frequency (mis-)alignment in J-difference GABA editing. J. Magn. Reson. Imaging 2013;38:970–975 doi: 10.1002/jmri.23923.
5. Henry P-G, van de Moortele P-F, Giacomini E, Nauerth A, Bloch G. Field-frequency locked in vivo proton MRS on a whole-body spectrometer. Magn. Reson. Med. 1999;42:636–642 doi: 10.1002/(SICI)1522-2594(199910)42:4<636::AID-MRM4>3.0.CO;2-I.
6. Zaitsev M, Speck O, Hennig J, Büchert M. Single-voxel MRS with prospective motion correction and retrospective frequency correction. NMR Biomed. 2010;23:325–332 doi: 10.1002/nbm.1469.
7. Bogner W, Gagoski B, Hess AT, et al. 3D GABA imaging with real-time motion correction, shim update and reacquisition of adiabatic spiral MRSI. Neuroimage 2014;103:290–302 doi: 10.1016/j.neuroimage.2014.09.032.
8. Waddell KW, Avison MJ, Joers JM, Gore JC. A practical guide to robust detection of GABA in human brain by J-difference spectroscopy at 3 T using a standard volume coil. Magn. Reson. Imaging 2007;25:1032–1038 doi: 10.1016/j.mri.2006.11.026.
9. Near J, Edden R, Evans CJ, Paquin R, Harris A, Jezzard P. Frequency and phase drift correction of magnetic resonance spectroscopy data by spectral registration in the time domain. Magn. Reson. Med. 2015;73:44–50 doi: 10.1002/mrm.25094.
10. Wilson M. Robust retrospective frequency and phase correction for single-voxel MR spectroscopy. Magn. Reson. Med. 2019;81:2878–2886 doi: 10.1002/mrm.27605.
11. Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 1998;11:266–272 doi: 10.1002/(SICI)1099-1492(199810)11:6<266::AID-NBM530>3.0.CO;2-J.
12. Mikkelsen M, Saleh MG, Near J, et al. Frequency and phase correction for multiplexed edited MRS of GABA and glutathione. Magn. Reson. Med. 2018;80:21–28 doi: 10.1002/mrm.27027.
13. Mikkelsen M, Tapper S, Near J, Mostofsky SH, Puts NAJ, Edden RAE. Correcting frequency and phase offsets in MRS data using robust spectral registration. NMR Biomed. 2020;33:e4368 doi: 10.1002/nbm.4368.
Figures