1985
Simultaneous Water and Lipid Suppression Using Chemical Selective Adiabatic Refocusing Pulses Echo Planar Spectroscopic Imaging (EPSI) at 7T
Guodong Weng1, Sulaiman Sheriff2, Claus Kiefer1, Irena Zubak3, Andrew A Maudsley2, and Johannes Slotboom1
1Institute for Diagnostic and Interventional Neuroradiology, Support Center for Advanced Neuroimaging (SCAN), University of Bern, Bern, Switzerland, 2Department of Radiology, University of Miami School of Medicine, Miami, FL, United States, 3Inselspital Bern and University Hospital, Bern, Switzerland
1Institute for Diagnostic and Interventional Neuroradiology, Support Center for Advanced Neuroimaging (SCAN), University of Bern, Bern, Switzerland, 2Department of Radiology, University of Miami School of Medicine, Miami, FL, United States, 3Inselspital Bern and University Hospital, Bern, Switzerland
Synopsis
To tackle the chemical shift displacement artifacts (CSDA), B1-inhomoheneity, water and fat suppression as well as specific absorption-rate (SAR) limitation at 7T spectroscopy, an EPSI-variant sequence is developed in this study. The conventional slice-selective refocusing pulse is replaced by a spectral-selective adiabatic 2π-pulse pair. The results show a 77% reduction of CSDA, homogeneous refocusing map, water suppression factor ≥ 1000 within an acceptable SAR both in vitro and in vivo measurement. The scan time for the whole brain is within 8 minutes. This new EPSI-variant sequence shows its high application potential clinical routine.
INTRODUCTION
At high magnetic field (≥7T) (i.) B1-inhomogeneity, (ii.) water and lipid signal-suppression and associated artifacts, (iii.) limitations on in-vivo specific absorption-rate (SAR), and (vi.) chemical-shift displacement artifact (CSDA) are four major factors that impose restrictions on application, interpretation, and quantification of the EPSI-data.1 However, the use of adiabatic (refocusing) pulses allow minimization of the effect of B1+-inhomogeneity 2 and, additionally, the SAR and bandwidth of these type of RF-pulses can be independently controlled by selection of appropriate RF-pulse parameters, and these pulses have therefore a clear advance over amplitude-modulated RF-pulse schemes.3 Finally, CSDA can be minimized by avoiding the use of spatial selective RF-pulses. In order to tackle all 4 points mentioned at the same time, we here propose to replace the slice selective (adiabatic) refocusing pulse (pair) in the EPSI pulse sequence 4 by a spectral-selective adiabatic-complex-secant-hyperbolic 2π-pulse pair 5 in our adapted EPSI-pulse scheme.METHODS
The changes we made in the EPSI-sequence.4 The slice-selective 180-degree refocusing pulse (Figure 1a) is replaced by two identical chemical shift-selective 180-degree secant hyperbolic adiabatic pulses $$$B_1^+(t) = Ω_0 \cdot sech(βt)^{1+\mu i}$$$ (Figure 1b). Specifically, the bandwidth (BW) of adiabatic pulses is set to 0.8 kHz (i.e. 2.7 ppm), and the carrier frequency was set at 3.0 ppm. The BW of the non-adiabatic excitation pulse is 5.5 kHz (18.5 ppm). In vitro tests were carried out in a spherical brain metabolite phantom (GE), and in vivo tests on one healthy volunteer and one patient (glioma). All measurements were performed on a 7T MAGNETOM Terra MR-scanner (Siemens, Germany) in clinical-mode using the 1Tx head coil.RESULTS AND DISCUSSION
The original EPSI-implementation 4 used a slice-selective Mao refocusing-pulse which is 1.25 kHz (limited by maximum RF-amplitude). The CSDA of the Mao-refocusing is 23.7%. For the non-selective adiabatic pulses, the CSDA is only determined by the excitation-pulse and is 5.4%. Therefore, the overall CSDA-error is reduced by approximately 1 - 5.4/23.7 = 77%. Due to the total absence of in-plane CSDA in case of the proposed chemical shift selective adiabatic refocusing, the amplitude variation of the spectral patterns of metabolites over the total excited 3D-volume is much smaller.Figure 2 shows the water signal integration maps using both EPSI-variants. Compared to using Mao-refocusing (Figure 2c), the water map of adiabatic pulses (Figure 2d) exciting the water is in good correspondence with the water-only excitation reference maps (Figure a-b): it indicates that despite the B1-inhomogeneity of the 1Tx-RF-head coil, signal losses due RF-inhomogeneity are almost absent by using the chemical selective adiabatic 2π-pulses. The histograms of the water amplitude over the volume are shown in Figure 2a'-d', and indicate that these 2π-pulses lead to significantly more homogeneous refocusing. The reason for this is that above a certain minimal RF-amplitude these pulses become B1+-insensitive and their flip-angle become nearly independent of the B1+-amplitude. Since the proposed 2π-pulses have a relatively small BW, they operate properly at lower amplitude-threshold and this result in lower SAR than using spatial selective refocusing without the CSDA-drawback.
Three adiabatic 2π-pulses with different BWs were applied to investigate their performance on a spherical phantom (Figure 3). Figure 3c shows nearly perfect water suppression is achieved with water signal suppression factor of ≥1000 compared to Figure 3a. In addition, due to the symmetry of BW around 3 ppm, equivalent suppression performance is expected for the lipid region (0.9 to 1.3 ppm).
Figure 4 shows water and creatine (both 3.7 and 3.0 ppm) integration maps as well as water reference maps using the Mao (Figure 4a'-d') and adiabatic (Figure 4a-d) pulses respectively. See the figure legend for further details.
In vivo studies of healthy brain tissue in two subjects (Figure 5b-c) show a high degree of agreement with the in vitro measurement (Figure 5a). The spectrum within the tumour region (Figure 5d) shows a clearly different pattern than the normal tissue of the same subject (Figure 5c). Furthermore, it is noteworthy that only k-space regridding and the Fourier transformation were performed during pre-and post-processing, and no water removal and baseline correction was used. Therefore, there are still various possibilities to improve the final results by post-processing, such as B0 and eddy current correction (ECC). The measurement time is about 8 minutes without parallel imaging techniques, which means that even a shorter scan time can be achieved by using GRAPPA or SENSE.
CONCLUSION
Single slab whole-brain EPSI allows the use of chemical selective small BW adiabatic 2π refocusing pulses, and is only possible to perform at high magnetic field strength (≥7T). It offers a way to tackle the B1+ inhomogeneity problem, SAR limitation and the CSDA simultaneously. Additionally, the proposed pulse sequence has excellent homogeneous water (fat) suppression. It has also been demonstrated that the proposed adiabatic 2π-pulses guarantee a uniform refocusing over both the selected chemical range and spatial volume. In vitro and in vivo studies confirmed the expected performance and capability for potential application clinical routine.Acknowledgements
This work was supported by the Swiss National Science Foundation grant number 182569. Additionally, this project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 813120. Initial development of the EPSI sequence was supported by NIH grant R01EB016064.References
- Maudsley AA, Andronesi OC, Barker PB, Bizzi A, Bogner W, Henning A, et al. Advanced magnetic resonance spectroscopic neuroimaging: Experts’ consensus recommendations. NMR Biomed [Internet]. 2020 Apr 29 [cited 2020 Dec 7]; Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/nbm.4309
- Slotboom J, Mehlkopf A., Bovée WMM. A single-shot localization pulse sequence suited for coils with inhomogeneous RF fields using adiabatic slice-selective RF pulses. J Magn Reson [Internet]. 1991 Nov [cited 2017 Sep 1];95(2):396–404. Available from: http://linkinghub.elsevier.com/retrieve/pii/002223649190229M
- Park JY, Garwood M. Spin-echo MRI using π/2 and π hyperbolic secant pulses. Magn Reson Med [Internet]. 2009 [cited 2020 Dec 7];61(1):175–87. Available from: /pmc/articles/PMC2688743/?report=abstract
- A. Ebel and A. A. Maudsley, “Improved spectral quality for 3D MR spectroscopic imaging using a high spatial resolution acquisition strategy,” Magn. Reson. Imaging, vol. 21, no. 2, pp. 113–120, 2003.
- Conolly S, Nishimura D, Macovski A. A selective adiabatic spin-echo pulse. J Magn Reson. 1989 Jun 15;83(2):324–34.
Figures

Figure1: (a.)
EPSI pulse sequence using spatial selective amplitude modulated excitation and
refocusing RF-pulses. (b.) Proposed EPSI variant of this abstract: the slice
selective Mao pulse typed refocusing pulse is replaced by a chemical shift
selective adiabatic complex hyperbolic-secant 2π-pulse pair. All the measurements
were performed with a volume of interest of 280 X 220 X 100 mm.
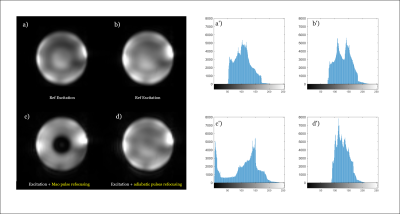
Figure 2: (a-d) water maps: comparison of non-adiabatic excitation
and refocusing using Mao pulses (a,c) with complex hyperbolic secant RF-pulses
(b,d). (a’-d’) corresponding histograms of the images (a-d) over the phantom
area. TE
= 82 ms, TR = 1500 ms, BW = 1.4 kHz with a resolution of 4.3 X 11 X 13.8 mm.
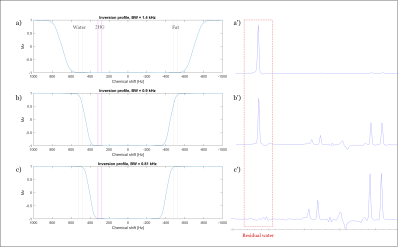
Figure 3: (a-c) The
simulated RF-pulse profiles of the adiabatic chemical shift selective 2π-pulses
(used as inversion pulse in the simulation for simplicity). (a’-c’) The corresponding brain metabolite phantom measurements. TE = 80 ms, TR = 1500 ms with a resolution of
4.3 X 11 X 13.8 mm.
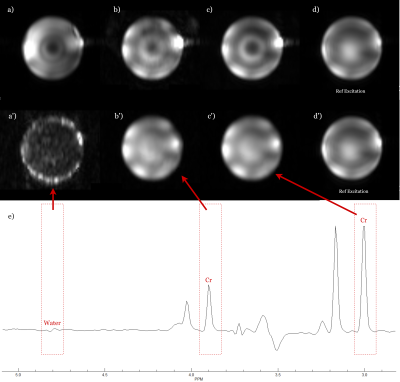
Figure 4: (a.) presents the noise-like water map which
emphasizes a superior homogeneous overall water suppression compared to (a').
The two creatine (b,c) and water reference maps (d) agree with each other,
while (4b'-d') show clearly different patterns. This proves that the proposed
adiabatic 2π-pulses guarantee a uniform refocusing over both the selected chemical range and spatial volume. TE = 82 ms, TR = 1500 ms, BW = 0.81 kHz with a
resolution of 4.3 X 11 X 13.8 mm.
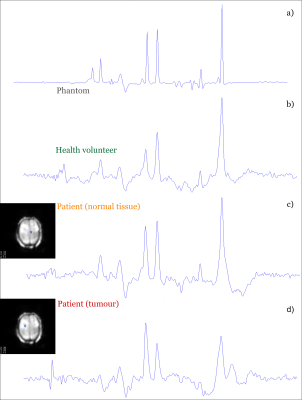
Figure 5: In vitro and in vivo measurement. TE = 82 ms, TR =
1551 ms, BW = 0.81 kHz with a resolution of 4.3 X 7.8 X 7.8 mm.