1984
Double Spin Echo Spectroscopic Imaging Using Optimized 3D Spectral-Spatial Pulses for Brain Studies at 10.5T1Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States
Synopsis
A parallel transmit optimized 3D spatial-spectral pulse is developed for spectroscopic imaging for brain studies at 10.5T with reduced SAR, intrinsic water suppression and field inhomogeneity mitigation. Phantom studies are used to compare the new method with a conventional approach.
Purpose
To overcome the limitations of current spin echo spectroscopic imaging methods to enable UHF studies in the human brain by providing localization, signal suppression and field compensation within allowable SAR limits.Methods
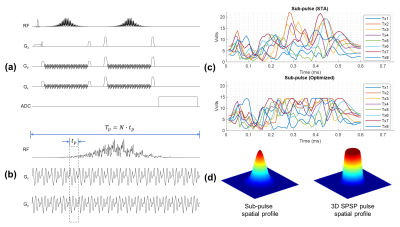
- Pulse Design and Spectroscopy SequenceA double spin echo sequence was adapted from the PRESS sequence (Figure 1.a), where the two refocusing pulses were replaced by 3D spectral-spatial (SPSP) pulses (Figure 1.b). No additional water or lipid suppression is needed due to the inherent spectral selectivity of the pulses. Compared to initial versions of such pulses (1, 2), additional consideration and optimization were required to support the spatial-spectral selectivity required for brain spectroscopy applications at UHF.
- Peak Amplitude Reduction
The optimal control method (3) was used to improve the pulse performance and reduce its peak amplitude. The sub-pulse was first designed by the spatial domain method and then scaled and fed into the optimal control method to achieve a large tip angle excitation, where the updated pulse waveform in each iteration was clipped by the specified maximum allowable amplitude (Figure 1.c).
- Spectral Bandwidth Improvement
To support the increased spectral bandwidth required to cover the metabolites of interest at 10.5T, an inhomogeneous target excitation profile was used to accelerate the sub-pulses while a homogeneous inversion was insured by driving the pulse with adequate power to reach adiabaticity, as shown in Figure 1.d. Such inhomogeneous target design can accommodate a reduced excitation k-space coverage enabling a shorter sub-pulse duration, which supports the required spectral bandwidth in the brain at 10.5T without introducing extensive under-sampling artifacts that usually arise when using a homogeneous target with such short pulse duration.
- Phantom Study
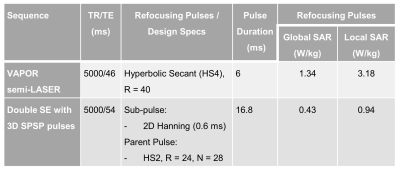
Spectroscopy acquisitions were performed on a Braino phantom at 10.5T using a home-made 8-channel transmit bumped dipole array (4,5). For double SE spectroscopy acquisitions, the 3D SPSP pulse was designed with the following specs: a 0.6 ms sub-pulse with a Hanning excitation profile, VOI 48x48 mm2 (defined by FWHM), using a spiral gradient with acceleration factor of 2, resolution 24 mm, maximum slew rate 180 T/m/s. The envelope was a hyperbolic secant adiabatic pulse (HS2) with 28 samples and a time-bandwidth product of 20. Power calibration was performed by acquiring flip angle mapping of the pTx sub-pulse to make sure to achieve the nominal flip angle. For comparison, spectra were acquired with semi-LASER (6) using HS4 pulses (6 ms, bandwidth 6.7 kHz) for refocusing and VAPOR (7) for water suppression. For semi-LASER, power calibration was performed using single-voxel-acquisitions arrayed over a series of excitation flip angles to determine the transmitter voltage needed. The HS pulses of choices for the two methods empirically had comparable chemical shift displacements.
- SAR Comparison
To calculate local SAR of the pulses, electromagnetic simulations were conducted on virtual-family human models “Duke” and “Ella” for the 8-channel bumped-dipole coil which will be used for future in vivo studies. Virtual observation points (8) from the Q-matrices were generated and used for local SAR calculation.
Results
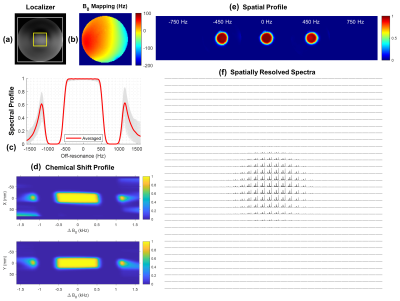
- Pulse PerformanceWith optimal control design, the peak amplitude of the pTx sub-pulse was effectively reduced by approximately 33%. By simulation the 3D SPSP pulse showed a 95% passband of around 920 Hz which covers a full 2 ppm of typical metabolites from myoinositol to NAA at 10.5T (Figure 2.c), with mild chemical shift displacement errors as shown in Figure 2.d, 2.e. Spatially resolved spectra were shown in Figure 2.f which matched the simulated spatial profile closely.
- Spectra Comparison
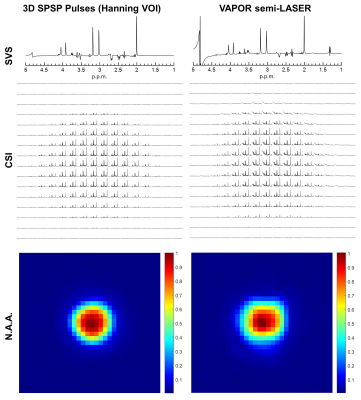
Both single-voxel-spectroscopy and spatially resolved spectra acquired with the proposed method and VAPOR semi-LASER were shown in Figure 3. Spectra were minimally processed including temporal exponential filtering, FFT, frequency shift correction and phase correction. The 3D SPSP pulses provided superior water suppression that is robust against B0 field non-uniformities. Metabolic mapping of NAA was performed considering that it was less impacted by water baseline in the case of semi-LASER.
- SAR Comparison
Global and local SAR of the two acquisitions were provided in Figure 4. The 3D SPSP pulses had significantly lower global and local SAR, making it possible to run spectroscopic imaging with a shorter TR at 10.5T, where the local SAR is especially more limiting at such high field strengths.
Conclusions
Our preliminary phantom results demonstrated the feasibility of optimizing spectroscopic acquisition with parallel transmit optimized 3D SPSP pulses at 10.5T providing advantages including reduced global and local SAR, faster acquisition, improved spectral suppression and robustness against field inhomogeneities, allowing a larger acquisition matrix to be used for the same scan time and thus enabling improved spatial resolution. Future studies will involve in vivo acquisitions to investigate localization and quantification performance in the human brain.Acknowledgements
No acknowledgement found.References
1. Jang A, Wu X, Auerbach EJ, Garwood M. Designing 3D selective adiabatic radiofrequency pulses with single and parallel transmission. Magn Reson Med 2018;79(2):701-710.
2. He X, Auerbach EJ, Garwood M, Wu X, Metzger GJ. Parallel Transmit Optimized Spiral Composite Adiabatic Pulses for 3D Spatial-Spectral Selectivity in Spectroscopy. Proc Intl Soc Mag Reson Med 2019;27:1060.
3. Xu D, King KF, Zhu Y, McKinnon GC, Liang ZP. Designing multichannel, multidimensional, arbitrary flip angle RF pulses using an optimal control approach. Magn Reson Med 2008;59(3):547-560.
4. Sadeghi-Tarakameh A, Adriany G, Metzger GJ, Lagore RL, Jungst S, DelaBarre L, Van de Moortele PF, Ugurbil K, Atalar E, Eryaman Y. Improving radiofrequency power and specific absorption rate management with bumped transmit elements in ultra-high field MRI. Magn Reson Med 2020;84(6):3485-3493.
5. Sadeghi-Tarakameh A, DelaBarre L, Lagore RL, Torrado-Carvajal A, Wu X, Grant A, Adriany G, Metzger GJ, Van de Moortele PF, Ugurbil K, Atalar E, Eryaman Y. In vivo human head MRI at 10.5T: A radiofrequency safety study and preliminary imaging results. Magn Reson Med 2020;84(1):484-496. 6. Scheenen TW, Klomp DW, Wijnen JP, Heerschap A. Short echo time 1H-MRSI of the human brain at 3T with minimal chemical shift displacement errors using adiabatic refocusing pulses. Magn Reson Med 2008;59(1):1-6.
7. Tkac I, Starcuk Z, Choi IY, Gruetter R. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med 1999;41(4):649-656. 8. Eichfelder G, Gebhardt M. Local specific absorption rate control for parallel transmission by virtual observation points. Magn Reson Med 2011;66(5):1468-1476.
Figures