1973
Deep learning-based reconstruction of highly-accelerated 3D MRI MPRAGE images1GE Research, Niskayuna, NY, United States, 2GE Research, Herzliya, Israel, 3GE Healthcare, Waukesha, WI, United States, 4Mayo Clinic College of Medicine, Rochester, MN, United States, 5Walter Reed National Military Medical Center, Bethesda, MD, United States, 6Uniformed Services University of the Health Sciences, Bethesda, MD, United States, 7GE Healthcare, Rochester, MN, United States
Synopsis
Three-dimensional (3D) MRI can achieve higher spatial resolution and signal-to-noise ratio than 2D MRI at the expense of long scan times. Recently, deep-learning (DL) techniques have been applied to reconstruction from highly undersampled data, resulting in significant scan accelerations. To assess clinical acceptability, we evaluated DL-based reconstruction on 3D MPRAGE data, using scores from image evaluation by neuroradiologists. Our DCI-Net method with reduction factor R=10 received scores higher than or equal to those of conventional parallel imaging with R=2.1. This implies the DL method can accelerate scans by an additional factor of 5 while maintaining comparable diagnostic image quality.
Introduction
Three dimensional (3D) MRI can achieve higher spatial resolution in the slice direction and higher signal-to-noise ratio (SNR) than 2D MRI at the expense of long scan times. Compressed sensing (CS) with parallel imaging enables image reconstruction from undersampled k-space data, resulting in significant scan acceleration. However, there are limits to the degree of acceleration that CS can achieve. Recently, deep-learning (DL) techniques1-4 have gained popularity for accelerating MRI while demonstrating better image quality than CS at the same reduction factors. Although there are a number of image quality metrics such as structural similarity index measure (SSIM)5 and neural network-based metrics6, the best way to assess clinical acceptability and usefulness of a reconstruction method is based on radiologists’ evaluation of diagnostic image quality.4 In this study, we evaluate DL-based accelerated reconstruction (reduction factor R=10) on T1-weighted MPRAGE, using scores from image evaluation by board-certified neuroradiologists, compared to the standard parallel imaging protocol (R=2.1).Methods
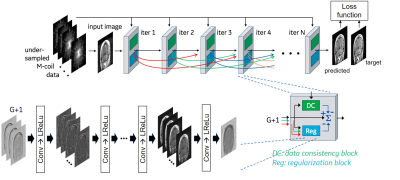
We evaluate three variants of DCI-Net (Densely Connected Iterative Network)3 that reconstruct images from Cartesian-undersampled multi-coil 3D k-space data. DCI-Net is an unrolled reconstruction method that consists of multiple iterative blocks, each of which includes a data-consistency unit and a convolutional unit for regularization, with dense skip-layer connections among iterations (Fig. 1). In the 2D DCI-Net, 2D convolution in the regularization unit is applied to the plane defined by the phase encoding and slice encoding directions. In the alternating 2D DCI-Net, 2D convolutions are applied, alternately, to the plane defined by the phase and slice encoding directions for 3 iterations, and then to the plane defined by the frequency and phase encoding directions for 1 iteration. In the 3D DCI-Net, 3D convolutions are applied. In the alternating 2D DCI-Net and the 3D DCI-Net, the convolution kernels were applied to 13 neighboring slices. See the caption of Figure 1 for network parameters used in this study.The DCI-Nets were trained on T1-weighted 3D brain scan data acquired using BRAVO (GE Healthcare) with retrospective undersampling (R=10) with variable-density Poisson-disc (VD-PD) sampling patterns. For training, 133 3D MRI datasets were used, and for validation, 15 were used. As a loss function for training, we used a contrast-weighted SSIM7 extended to complex-valued images where the exponents for the luminance, contrast and structure comparison functions were 0.3, 1 and 0.3, respectively.
For testing, 3D sagittal MPRAGE data (1 mm isotropic) were acquired on the high-performance 3.0 T MAGNUS head-gradient system8 (200 mT/m and 500 T/m/s gradients). Five subjects were recruited under an IRB-approved protocol and provided written informed consent. For each subject, the MPRAGE data were retrospectively undersampled with net R=10 (VD-PD), and 3 image volumes were reconstructed using the 3 DCI-Net methods. Baseline images were reconstructed by Autocalibrating Reconstruction for Cartesian imaging (ARC) with net R=2.1. For each subject, 4 image volumes (ARC, 2D DCI-Net, alternating 2D DCI-Net and 3D DCI-Net) were randomly arranged and scored by 3 board-certified neuroradiologists who were blinded to reconstruction methods. Image scoring was based on consensus reading, using a 5-point Likert scale for 8 scoring categories: SNR, artifacts, gray/white matter contrast, resolution/sharpness, deep gray, cerebellar vermis, anterior commissure, and overall quality. For the Likert scale, 1 was poor, and 5 was excellent. The assessments of the deep gray, cerebellar vermis, and anterior commissure categories focused on scoring anatomies with fine features that were thought to be vulnerable to artifacts such as blurring.
Results
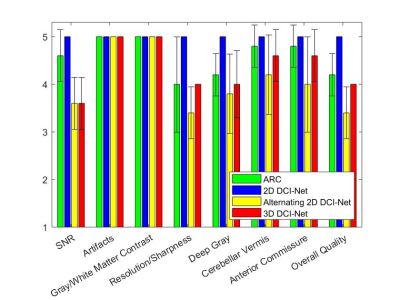
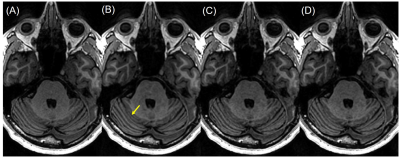
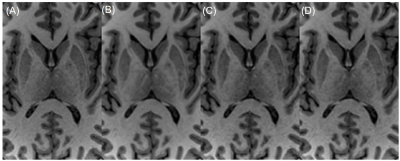
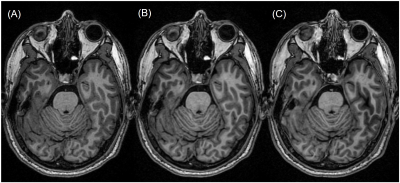
Figure 2 shows the mean scores over 5 subjects, comparing the standard ARC (R=2.1) and the three DCI-Net variants (R=10). The 2D DCI-Net received scores higher than or equal to those for the other methods including the baseline ARC. All the methods were free of artifacts and showed excellent gray/white matter contrast. Figures 3 and 4 show example images for comparison. The 2D DCI-Net showed better recovery of the cerebellar vermis (Fig. 3) and the deep gray region (Fig. 4) than the other methods.The 2D DCI-Net was also tested in prospective image acquisitions and compared to the fully sampled and the retrospectively undersampled (Fig. 5). With an R=9.9 acceleration, the scan time was 1 min 24 s compared to 13 min 33 s for the full data acquisition, realizing a substantial time saving.
Discussion
The 2D DCI-Net (R=10) was better than or comparable to the standard ARC (R=2.1) in all scoring categories. This implies the DL method can accelerate 3D MRI scans by an additional factor of 5 compared to standard parallel imaging while maintaining comparable diagnostic image quality. A recent study on 2D knee MRI also found that readers preferred DL-accelerated images than standard clinical images.4The 2D DCI-Net was better than or equal to the 3D DCI-Net in image quality scores probably because of fewer iterations, kernels and skip connections being used in the 3D DCI-Net due to memory limitations. Given that 3D convolutions have a potential for better performance than 2D convolutions, we may revisit the 3D DCI-Net when hardware/software advances permit the practical barriers to be avoided.
Conclusion
The 2D DCI-Net enables acceleration of 3D MPRAGE scans with R=10. The image quality scores for the 2D DCI-Net (R=10) were comparable to those for standard parallel imaging (R=2.1).Acknowledgements
This work was supported in part by CDMRP under Grant W81XWH-16-2-0054 and by NIH under Grant U01EB024450.References
1. Hammernik K, Klatzer T, Kobler E, et al. Learning a variational network for reconstruction of accelerated MRI data. Magn Reson Med 2018;79(6):3055-3071.
2. Schlemper J, Caballero J, Hajnal JV, et al. A deep cascade of convolutional neural networks for dynamic MR image reconstruction. IEEE Trans Med Imaging 2018;37(2):491-503.
3. Malkiel I, Ahn S, Slavens Z, et al. Densely connected iterative network for sparse MRI reconstruction. ISMRM 2018, p. 3363.
4. Recht MP, Zbontar J, Sodickson DK, et al. Using deep learning to accelerate knee MRI at 3T: Results of an interchangeability study. Am J Roentgenol. 2020;215(6):1421-1429.
5. Wang Z, Bovik AC, Sheikh HR, et al. Image quality assessment: from error visibility to structural similarity. IEEE Trans Image Process 2004;13(4):600-612.
6. Johnson J, Alahi A, Li F. Perceptual losses for real-time style transfer and super-resolution. ECCV 2016.
7. Ahn S, Menini A, McKinnon G, et al. Contrast-weighted SSIM loss function for deep learning-based undersampled MRI reconstruction. ISMRM 2020.
8. Foo TKF, Tan ET, Vermilyea ME, et al. Highly efficient head‐only magnetic field insert gradient coil for achieving simultaneous high gradient amplitude and slew rate at 3.0T (MAGNUS) for brain microstructure imaging. Magn Reson Med. 2020;83(6):2356-2369.
Figures