1971
Cardiac Functional Analysis with Cine MRI via Deep Learning Reconstruction1United Imaging Intelligence, Cambridge, MA, United States, 2UIH America, Inc., Houston, TX, United States, 3United Imaging Healthcare, Shanghai, China
Synopsis
Retrospectively gated cine (retro-cine) MRI is the clinical standard for cardiac functional analysis. To the best of our knowledge, this is the first work to evaluate the cine MRI with deep learning reconstruction for cardiac function analysis and compare it with other conventional methods. The cardiac functional values obtained from cine MRI with deep learning reconstruction are consistent with values from clinical standard retro-cine MRI.
Introduction
Cardiac functional analysis is crucial for the diagnosis and treatment of cardiovascular diseases (CVD)1. It often involves measuring the end-diastolic volume (EDV), end-systolic volume (ESV) and ejection fraction (EF) for the left and right ventricles from a series of cardiac images. Conventional segmented, retrospectively gated cine (retro-cine) MRI, the gold standard for cardiac MR functional analysis, provides dynamic information of the heart motion that is critical for function evaluation2. Multiple slices (e.g., 8-10 short-axis slices) are typically needed to achieve whole heart coverage and therefore multiple breath-holdings are required. Retro-cine may suffer from poor image quality resulting from the inconsistent breath-holding position, prolonged exam time due to its segmented acquisition, and limited use in patients challenged by long breath-holding.Compressed sensing (CS) is an emerging fast MR imaging technique with a specifically designed acquisition and reconstruction strategy3. The potential of CS-cine in cardiac functional analysis with the highly undersampled acquisition (8X-15X acceleration) has been demonstrated4. However, its clinical use is hampered by long reconstruction time due to the nature of iterative algorithms. This challenge could be further amplified in the reconstruction of multiple slices (e.g., one-hour reconstruction time4).
Deep learning (DL) based methods have been proposed for the reconstruction of highly undersampled MRI data and show superior image quality and magnitude faster reconstruction time than CS-based methods5. Nevertheless, it remains unclear whether DL reconstruction is suitable for cardiac function analysis. To address the above question, in this study we evaluate and compare the cardiac functional values (EDV, ESV and EF for LV and RV, respectively) obtained from highly accelerated MRI acquisition using DL based reconstruction algorithm (DL-cine) with values from CS-cine and conventional retro-cine. To the best of our knowledge, this is the first work to evaluate the cine MRI with DL reconstruction for cardiac function analysis.
Methods
Data collection: We collected total 216 cine MRI data from seven volunteers and one patient with cardiomegaly using a bSSFP sequence on a clinical 3T scanner (uMR 790 United Imaging Healthcare, Shanghai, China) with the approval of local IRB. For each subject, we acquired three datasets with different protocols and reconstruction methods, namely, retro-cine, CS-cine, DL-cine.The imaging parameters for retro-cine were: imaging matrix: 224 x 199 x 9(slices), TR/TE = 3.13/1.48 ms, FOV = 360 x 320 mm2, slice thickness = 8 mm, flip angle = 43°~50°, bandwidth = 1200 Hz/pixel, temporal resolution was 34ms, reconstructed cardiac phase=25. The k-space was prospectively undersampled with net reduction factor of 1.8. The imaging parameters for CS-cine and DL-cine were: imaging matrix: 224 x 199 x 9 (slices), TR/TE = 2.89/1.34 ms, bandwidth = 1200 Hz/pixel, 15 phase encoding lines were collected for each cardiac phase, with a temporal resolution of 43.4 ms, FOV = 360 x 320 mm2, slice thickness = 8 mm, flip angle = 50°.The k-space was prospectively undersampled using a lookup table using variable Latin Hypercube Sampling6 with a net reduction factor of 15.
Image reconstruction: For retro-cine, parallel imaging was used for reconstruction. For CS-cine, temporal sparsity regularization was used in the CS reconstruction framework. For DL-cine, we adopted the convolutional recurrent neural network model, Res-CRNN5, which takes into account the dynamic information and reconstruct cardiac cine images. The model was pre-trained using retro-cine data5 and directly applied to the cine data in this study.
Cardiac functional analysis: The UNet-based segmentation model7 was first used to provide preliminary LV myocardium, LV blood pool and RV blood pool segmentation. The model was trained on retro-cine data from ACDC8, and applied to all cine data in this study. The segmentation were then manually adjusted. LV and RV volumes were calculated from the multi-slice segmentation using Simpson's rule. EDV, ESV were obtained as the maximum and minimum points on the volume curve, respectively. EF was calculated as EF = (EDV-ESV)/ESV.
Results
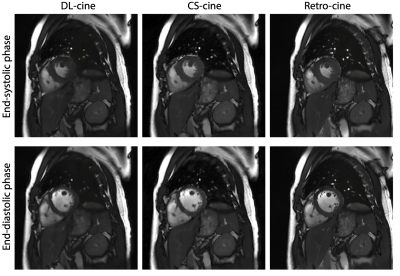
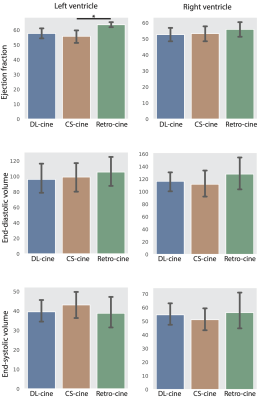
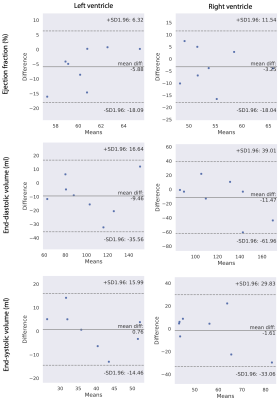
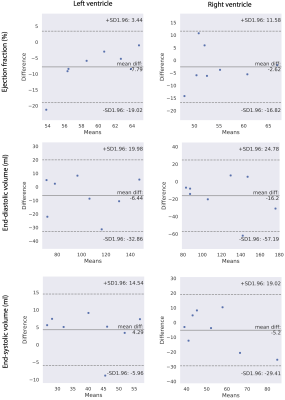
Figure 1 shows the examples of the reconstructed images from DL-cine, CS-cine and retro-cine MRI. All methods show good image quality. Figure 2 shows the average values of EF, EDV and ESV for LV and RV across different methods. No statistically significant difference was found between DL-cine and retro-cine for EF, EDV and ESV (all p>0.05 by Wilcoxon signed-rank test). CS-cine also shows similar results with retro-cine except for lower average values of LV EF than retro-cine (p < 0.05). The Bland-Altman plots (Figure 3 and 4) indicate that DL-cine has a good agreement with retro-cine and it is better than CS-cine since a few values are out of 95% limits of agreement in the latter.Discussion and Conclusion
This is the first work that compares cine MRI using DL reconstruction with retro-cine for cardiac functional analysis. For EF, EDV and ESV, no statistically significant difference was found between DL-cine and retro-cine MRI, which is the clinical standard for cardiac functional analysis. Compared to retro-cine, DL-cine has the advantage of faster data acquisition. Furthermore, DL-cine has faster reconstruction speed as well as superior image quality than CS-cine5. This suggests that cine MRI with DL reconstruction is suitable for clinical usage on cardiac functional analysis with fast data acquisition and image reconstruction, which can be especially beneficial for patients who have difficulties holding their breath.Acknowledgements
No acknowledgement found.References
1. Theodoros D Karamitsos, Jane M Francis, Saul Myerson, Joseph B Selvanayagam, and Stefan Neubauer. The role of cardiovascular magnetic resonance imaging in heart failure. Journal of the American College of Cardiology, 54(15):1407–1424, 2009.
2. James CC Moon, Christine H Lorenz, Jane M Francis, Gillian C Smith, and Dudley J Pennell. Breath-hold flash and fisp cardiovascular MR imaging: left ventricular volume differences and reproducibility. Radiology, 223(3):789–797, 2002.
3. Oren N Jaspan, Roman Fleysher, and Michael L Lipton. Compressed sensing MRI: a review of the clinical literature. The British journal of radiology,88(1056):20150487, 2015.
4. Li Feng, Monvadi B Srichai, Ruth P Lim, Alexis Harrison, Wilson King, Ganesh Adluru, Edward VR Dibella, Daniel K Sodickson, Ricardo Otazo, and Daniel Kim. Highly accelerated real-time cardiac cine MRI using k–t sparse-sense. Magnetic resonance in medicine, 70(1):64–74, 2013.
5. Eric Z Chen, Xiao Chen, Jingyuan Lyu, Yuan Zheng, Terrence Chen, Jian Xu, and Shanhui Sun. Real-time cardiac cine MRI with residual convolutional recurrent neural network. ISMRM. 2019.
6. Jingyuan Lyu, Yu Ding, Jiali Zhong, Zhongqi Zhang, Lele Zhao, Jian Xu,Qi Liu, Ruchen Peng, and Weiguo Zhang. Toward single breath-hold whole-heart coverage compressed sensing MRI using variable spatial-temporal latin hypercube and echo-sharing (valas). ISMRM, 2019.
7. Olaf Ronneberger, Philipp Fischer, and Thomas Brox. U-net: Convolutional networks for biomedical image segmentation. International Conference on Medical image computing and computer-assisted intervention, pages 234–241. Springer, 2015.
8. Olivier Bernard, Alain Lalande, Clement Zotti, Frederick Cervenansky, Xin Yang, Pheng-Ann Heng, Irem Cetin, Karim Lekadir, Oscar Camara, Miguel Angel Gonzalez Ballester, et al. Deep learning techniques for automatic MRI cardiac multi-structures segmentation and diagnosis: is the problem solved?IEEE transactions on medical imaging, 37(11):2514–2525, 2018
Figures