1963
Deep Learning Image Reconstruction from Incomplete Fast Spin Echo MR Data1Laboratory of Biomedical Imaging and Signal Processing, The University of Hong Kong, Hong Kong, China, 2Department of Electrical and Electronic Engineering, The University of Hong Kong, Hong Kong, China, 3Department of Electrical and Electronic Engineering, Southern University of Science and Technology, Shenzhen, China
Synopsis
Fast spin echo (FSE) is the most commonly used multi-shot sequence in clinical MRI. In this study, we propose to acquire single-channel FSE data with incomplete number of shots (TRs), and reconstruct such periodically undersampled k-space data using a deep learning approach. The results demonstrate that the proposed method can effectively remove the aliasing artifacts and recover the high frequency information without noise amplification, enabling a FSE acceleration that can be readily implemented in practice.
Introduction
Fast spin echo (FSE) is the most commonly used multi-shot sequence in clinical MRI. One simple and intuitive way to accelerate FSE imaging is to acquire incomplete FSE data by simply reducing number of shots (i.e., TRs). However, reconstructing high-quality images from such incomplete and periodically undersampled FSE data is challenging to conventional MR reconstruction methods due to the strong aliasing artifacts. Recently, deep neural networks have emerged as a powerful approach for MR image reconstruction, noise suppression and artifacts removal1. In this study, we propose a deep learning image reconstruction method for the incomplete single-channel FSE data with partial shots. The results demonstrate that the method could effectively remove the aliasing artifacts and recover the high frequency information without noise amplification.Method
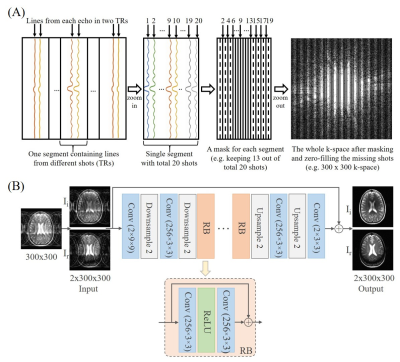
Proposed Sampling Pattern and ModelThe proposed sampling pattern for incomplete single-channel FSE data acquisition with partial shots (TRs) is illustrated in Figure 1(A). For example, to exploit k-space conjugate symmetry, an easy-to-implement sampling pattern or mask is utilized to include odd and even phase encoding lines on two sides, respectively, and a few consecutive central k-space lines. As depicted in Figure 1(B), we designed a residual neural network (ResNet)2 for image reconstruction from incomplete FSE data. It consists of two convolutional layers with downsampling, multiple residual blocks, and two convolutional layers with upsampling. Each residual block contains two convolutional layers with a rectified linear unit in between. The real and imaginary parts of complex images are inputted into the network as two separate channels. Similarly, the outputs are the real and imaginary parts of the predicted images in the corresponding two channels.
Model Training and Testing
The proposed method was evaluated with the recently released fastMRI T2-weighted multi-coil brain dataset3, which included a set of raw k-space datasets acquired by 2D FSE from 2500 subjects. The multi-channel complex images were combined using virtual body coil method4 to simulate single-channel data while preserving phase information. Then 16 consecutive central axial images were extracted from each subject, resized to 300×300 images with echo train length (ETL) and total shot numbers reorganized to 15 and 20, respectively. The resulting 40000 images were randomly divided for training (70%), validation (10%) and testing (20%). Incomplete FSE data was prepared by discarding the different number of shots, yielding data containing 13, 12 and 11 shots out of total 20 shots with ETL=15. In addition, the data was also prepared for 7 out of 12 total shots with ETL=25.
The training was carried out by optimizing the mean absolute error using Adam with a batch size of 256 and an initial learning rate of 2×10-4. After every 2 epochs, we reduced the learning rate by 20%. We trained the proposed ResNet model for 80 iterations, which took approximately 16 hours on an NVIDIA RTX 8000 GPU.
Testing with Separate Clinical Data
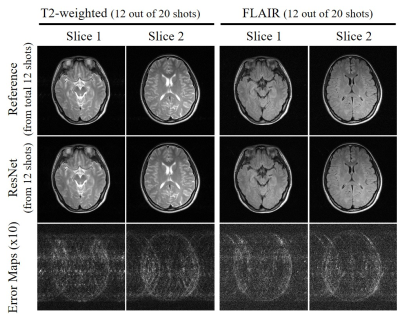
The proposed method was also evaluated with brain MR data acquired on a separated 3T Philips scanner using an 8-channel head coil. FSE data was acquired with ETL=15, FOV= 240×240mm2, acquisition matrix size 300×300 and TE/TR=86/3000ms, and TE/TI/TR=135/2500/8000ms for T2-weighted and FLAIR imaging, respectively. Quantitative measurement was performed by comparing the peak signal-to-noise ratio (PSNR) and structural similarity index (SSIM).
Results
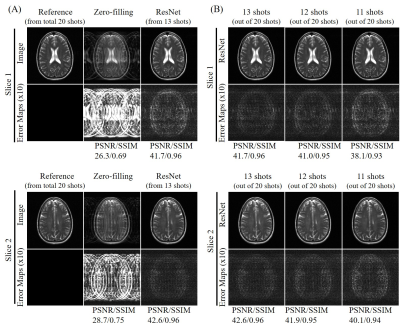
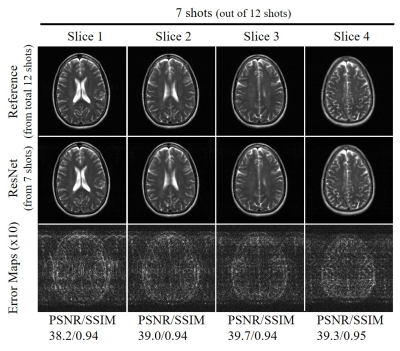
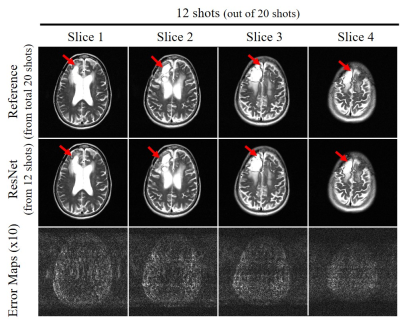
Figure 2 shows the typical reconstruction results by the proposed ResNet model for incomplete single-channel FSE data with 13, 12 and 11 shots out of total 20 shots, respectively. The trained model effectively removed the aliasing artifacts and recovered the high frequency information without noticeable blurring at different undersampling levels using various partial shots. Figure 3 shows the performance of ResNet model in reconstructing from partial shot number 7 (out of total 12 shots with ETL=25). The results clearly indicated the robustness of the proposed ResNet model in consistently removing the aliasing artifacts and recovering the high frequency information with different acquisition parameters, providing significant acceleration. In Figure 4, we applied the trained model to the incomplete single-channel FSE MR data in presence of a brain lesion with 12 out of total 20 shots. The aliasing artifacts were successfully removed without noise amplification and the lesion remained to be truthful in the reconstructed image. Furthermore, we applied the model to MR brain images acquired on a separated 3T Philips MRI scanner. The reconstruction results of T2-weighted FSE and FLAIR brain images are shown in Figures 5(A) and 5(B), respectively, further demonstrating the robustness and effectiveness of the proposed method.Discussion and Conclusions
This study presented a new FSE acquisition and deep learning reconstruction approach to acquire and reconstruct incomplete single-channel FSE data with significantly reduced number of shots (TRs). The results indicated that the method could effectively remove the aliasing artifacts and recover high frequency information without noise amplification for various FSE acquisition parameters. The trained models were also shown to be applicable to reconstruct other brain datasets that were of different contrasts and acquired on a different MRI scanner. In the future, we will (1) further optimize the proposed model and FSE undersampling patterns; (2) apply our approach to 3D FSE to achieve a larger acceleration factor by reducing both in-plane and through-plane phase encoding steps; and (3) combine with existing multi-channel parallel imaging methods.Acknowledgements
This study was supported by Hong Kong Research Grant Council (R7003-19, C7048-16G, HKU17112120, HKU17103819 and HKU17104020), Guangdong Key Technologies for Treatment of Brain Disorders (2018B030332001), and Guangdong Key Technologies for Alzhemier’s Disease Diagnosis and Treatment (2018B030336001).References
1. Mazurowski, M. A., Buda, M., Saha, A., & Bashir, M. R. Deep learning in radiology: An overview of the concepts and a survey of the state of the art with focus on MRI. Journal of magnetic resonance imaging. 2019; 49(4), 939-954.
2. He, K., Zhang, X., Ren, S., & Sun, J. Deep residual learning for image recognition. In Proceedings of the IEEE conference on computer vision and pattern recognition. 2016; 770-778.
3. Jure Zbontar, Florian Knoll, Anuroop Sriram, Matthew J. Muckley, Mary Bruno, Aaron Defazio, Marc Parente,Krzysztof J. Geras, Joe Katsnelson, Hersh Chandarana, Zizhao Zhang, Michal Drozdzal, Adriana Romero, Michael Rabbat, Pascal Vincent, James Pinkerton, Duo Wang, Nafissa Yakubova, Erich Owens, C. Lawrence Zitnick,Michael P. Recht, Daniel K. Sodickson, and Yvonne W. Lui. fastMRI: An open dataset and benchmarks for accelerated MRI. 2018.
4. Buehrer, M., Boesiger, P., & Kozerke, S. Virtual body coil calibration for phased-array imaging. In Proceedings of the 17th Annual Meeting ISMRM. 2009; 760.
Figures