1957
Reconstruction of Whole-Heart Cardiac Radial MRI using Neural Network Transfer Learning Approach1Service of Radiology, Geneva University Hospitals and Faculty of Medicine, University of Geneva, Geneva, Switzerland, 2Medical Image Processing Research Group (MIPRG), Deptt. of Electrical & Computer Engineering, COMSATS University Islamabad, Islamabad, Pakistan
Synopsis
In a clinical setting, multiple breath-hold, multi-slice, ECG-gated cine MR (CMR) Cartesian acquisition is a gold standard. Multiple breath-holds in standard CMR acquisition can result in slice-misalignment due to inconsistent breath-hold positions and it forces long exam time. To reduce CMR scan time and to avoid slice-misalignment, under-sampled non-Cartesian (NC) trajectories are useful but lead to artifacts. This paper proposes U-Net based transfer-learning approach with NUFFT (NUFFT TL-Net) to reconstruct artifact-free whole heart, radial CMR images. The preliminary experiments show improved performance of the proposed NUFFT TL-Net both visually and in terms of evaluation parameters than contemporary NUFFT U-Net.
Introduction
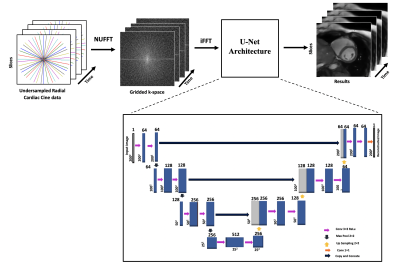
Cardiac cine MRI (CMR) is a clinical imaging technique used for a noninvasive diagnosis of heart function1. Multiple breath-hold, multi-slice, ECG-gated Cartesian CMR acquisition is a benchmark in a clinical setting. With the possibility of different breath-hold positions within an acquisition, this standard CMR can cause slice-misalignment and requires a long exam time2. Undersampled non-Cartesian (NC) trajectories help to accelerate the MR scan time to avoid slice-misalignment. NC trajectories are robust to motion and allow reduced field-of-view without wraparound artifacts, however, an additional gridding step is needed before applying inverse Fourier Transform (iFFT)3. Non-Uniform Fast Fourier Transform (NUFFT)4 is a well-known gridding method that uses min-max interpolation in k-space to map the NC onto a Cartesian grid. This paper proposes a U-Net based transfer learning approach with NUFFT (NUFFT TL-Net) to reconstruct the artifact-free whole heart, multi-slice radial CMR images using end-to-end fine-tuning5. The proposed NUFFT TL-Net reconstruction results are compared with the conventional NUFFT U-Net6 framework at acceleration factors of (AF) 7 and 13.Method
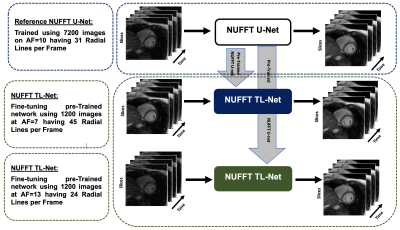
Figure-1 shows the schematic diagram for the pre-trained NUFFT U-Net at AF=10 and Figure-2 presents the proposed NUFFT TL-Net framework for AF 7 and 13. The pre-trained NUFFT U-Net6 is initially trained at AF=10 using a training set of 7200 CMR images, splitted into 6480 training and 720 validation images during the training phase. The pre-trained NUFFT U-Net has 14 convolution layers; initial weights are zero with a standard deviation of 0.05 and weight decay factor of 0.1. The loss function is minimized via RMSProp optimizer with a learning rate of 1×10-4. For the proposed NUFFT TL-Net, the pre-trained NUFFT U-Net is end-to-end fine-tuned using 1200 radial undersampled images for AF= 7 and 13, individually, using 150 epochs with a stopping criterion of 100 epochs using a learning rate of 3×10-9. The pre-trained NUFFT U-Net and the proposed NUFFT TL-Net networks were trained on Intel(R) Xeon (R) CPU,128GB RAM, and GPU NVIDIA GeForce GTX 1080Tei using TensorFlow as a backend5 with Python 3.8. The proposed method was tested on retrospectively under-sampled 1450 images (4 patients’ data) at AF=7 and13 with 45 and 24 spokes per image. Image quality was evaluated using AP, RMSE PSNR, SSIM, Sharpness Index (SI), and Image Correlation (IC)3. Also, image quality is blindly assessed by a cardiac MRI expert based on the artifacts in the reconstructed images (Ranked 1-5: Absent-Severe). For result analysis, a standard student t-test was used.Results and Discussion
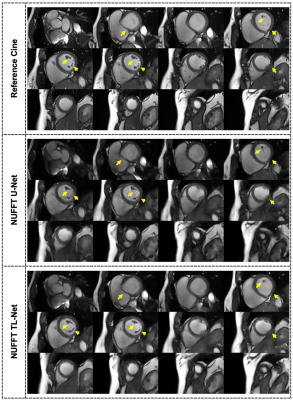
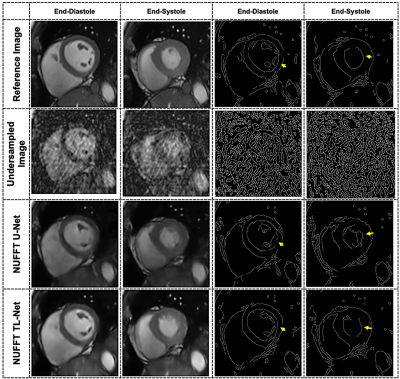
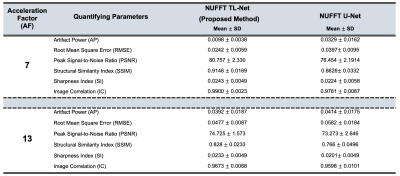
Figure-3 presents the radial cine, short-axis multi-slices reconstruction results at AF=13 for a single patient images using the proposed NUFFT TL-Net method and conventional NUFFT U-Net6. The myocardium wall distortion is seen in the NUFFT U-Net while NUFFT TL-Net preserves maximum details. Figure-4 shows the middle slice short-axis End-diastole and End-Systole reconstructed images of the same patient at AF=13; the corresponding edge images provide reconstruction quality assessment. The qualitative visual artifact average score (by the expert cardiologist) for the proposed method was 2.13±0.69 while the NUFFT U-Net was 2.87±0.69 at AF=7 &13 (p< 0.05). Table-1 gives the quantitative image quality assessment using a student t-test with p-value < 0.05. On average, the proposed NUFFT TL-Net gives 34% improvement in AP, 26% improvement in RMSE, 4% improvement in PSNR, 7% improvement in SSIM, 12% improvement in SI, and 1% improvement in IC than NUFFT U-Net in our experiments.Conclusion
Visual artifact score and quantitative results confirm that the proposed NUFFT TL-Net provided better results than NUFFT U-Net. In addition, the proposed method did not require any training of the deep convolution neural network for each AF from scratch, which significantly reduces the training time from 18 hours to 30 minutes.Acknowledgements
This work was partially funded by the COST Action (European Cooperation in Science and Technology): Magnetic Resonance Imaging Biomarkers for Chronic Kidney Disease (PARENCHIMA)References
1. J. Jeudy and C. S. White, “Cardiac Magnetic Resonance Imaging: Techniques and Principles,” Semin. Roentgenol., vol. 43, no. 3, pp. 173–182, Jul. 2008.
2. H. Haji-Valizadeh et al., “Validation of highly accelerated real-time cardiac cine MRI with radial k-space sampling and compressed sensing in patients at 1.5T and 3T,” Magn. Reson. Med., vol. 79, no. 5, pp. 2745–2751, 2018.
3. I. Aslam, F. Najeeb, and H. Omer, “Accelerating MRI Using GROG Gridding Followed by ESPIRiT for Non-Cartesian Trajectories,” Appl. Magn. Reson., vol. 49, no. 1, pp. 107–124, Jan. 2018.
4. J. A. Fessler and B. P. Sutton, “Nonuniform fast fourier transforms using min-max interpolation,” IEEE Trans. Signal Process., vol. 51, no. 2, pp. 560–574, Feb. 2003.
5. M. Arshad, M. Qureshi, O. Inam, and H. Omer, “Transfer learning in deep neural network based under-sampled MR image reconstruction,” Magn. Reson. Imaging, vol. 76, pp. 96–107, Feb. 2021.
6. L. Fan et al., “Rapid dealiasing of undersampled, non‐Cartesian cardiac perfusion images using U‐net,” NMR Biomed., vol. 33, no. 5, May 2020.
Figures