1943
Maturation and degeneration of the human cerebrum across the adult lifespan1Laboratory of Clinical Investigation, National Institute on Aging, Baltimore, MD, United States
Synopsis
Using myelin water fraction (MWF) and DTI, we investigated age- and sex-related differences in brain maturation and degeneration in a large cohort of unimpaired participants. We observed quadratic relationships between MWF or DTI indices and age, suggesting that brain maturation continues until middle age followed by a phase of rapid degeneration afterward. Sexual dimorphism in these processes was not significant in most cerebral regions studied. Finally, we observed weak-to-moderate correlations between DTI indices and MWF indicating that these indices could not serve as proxies of myelin content while highlighting the value of using multiple quantitative MRI metrics in clinical investigation.
Introduction
Postmortem studies of human brain have revealed lifespan differences in white matter (WM) microstructure, including changes in myelin content and axonal density or dispersion (1, 2). However, histological postmortem studies cannot be performed in real-time on living subjects and there is therefore limited ability to perform correlative studies with cognitive performance and treatment. Therefore, characterizing age-related differences in-vivo is essential for identifying biomarkers of tissue microstructure, distinguishing age-dependent changes from neurodegeneration, and evaluating therapeutic interventions. Here, we investigated age and sex differences in WM microstructure using diffusion tensor imaging (DTI) and myelin water fraction (MWF) imaging, a direct and specific measure of myelin content (3, 4). Our study is conducted on a large cohort of well-documented cognitively normal subjects spanning a wide-age range. We also investigated whether the DTI indices, especially radial diffusivity (RD) and fractional anisotropy (FA), could serve as proxies to probe differences in myelin content; this is a research topic of outstanding controversial discussions (5-8).Methods
Study cohort and data acquisitionThe study cohort consisted of 147 subjects (53.7 ± 21.2 years, 63 women) spanning the age range between 21 and 94 years. All subjects have undergone the BMC-mcDESPOT imaging protocol for MWF imaging (9-11). Among them, 132 (52.4 ± 21 years, 58 women) have also undergone our DTI imaging protocol for RD, FA, mean diffusivity (MD), and axial diffusivity (AxD) imaging.
Image processing
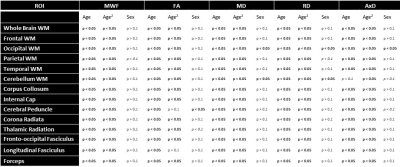
For each subject, a whole-brain MWF map was generated using the BMC-mcDESPOT analysis (1-3). Furthermore, corresponding FA, RD, MD, and AxD maps were calculated from the eigenvalues derived from the corresponding DTI dataset using the DTIfit tool implemented in FSL (12). All derived parameter maps were nonlinearly registered to the MNI space using FSL (12). Finally, fourteen cerebral WM regions-of-interest (ROIs) were defined from MNI (Fig. 2&4).
Statistical analyses
To investigate age and sex effects on MWF, RD, FA, MD, or AxD in each ROI, linear regression analyses were performed using the mean value of MWF, RD, FA, MD, or AxD within each ROI as the dependent variable, and sex, age, and age2 as independent variables.
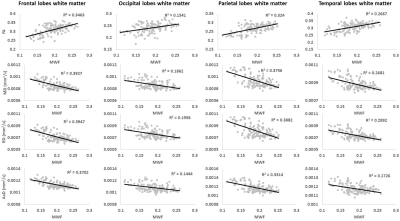
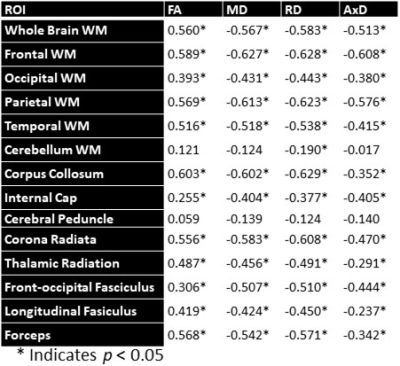
It is widely assumed that FA and RD could serve as specific metrics to probe changes in myelin content with neurodevelopment or pathology. Here, for each ROI, we tested this assumption using Pearson correlation by correlating each derived DTI index to MWF, which represents a more specific and sensitive measure of myelin content (3, 4).
Results & Discussion
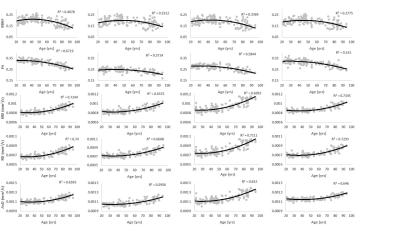
Figure 1 shows MWF and DTI indices as a function of age for representative WM regions. The best-fit curves indicate that while the fundamental quadratic relationships between each investigated parameter and age were consistent across ROIs, these patterns differed in detail among regions. The quadratic effect of age, that is, age2, on all investigated parameters was significant in most cerebral structures studied (Fig. 2). Faizy and colleagues and Billiet and colleagues have shown linear or no trends between MWF and age (5, 13), while Arshad and colleagues (6) and our previous work (14), conducted on much smaller study cohorts as compared to the study cohort in this work, have demonstrated quadratic associations between MWF and age. We believe that these quadratic, more physiologically plausible trends reflect continuing brain myelination until middle age followed by a rapid decline afterward. Our current results, obtained on a substantially larger study cohort, provide further evidence and support to this nonlinear relationship. Furthermore, our results indicate that all DTI indices follow quadratic associations with age. Although these results disagree with Arshad and colleagues’ observations of no trend (6), they support others’ observations of nonlinear relationships (15-17). Finally, sex effect on MWF and DTI indices was not significant. Literature regarding sexual dimorphism in white matter microstructure is sparse, requiring further investigation.Figure 3 shows examples of the correlation plots between DTI indices and MWF. Pearson correlations across the 14 ROIs studied and over all participants demonstrate significant correlations (Fig. 4). Specifically, FA vs. MWF exhibited significant positive correlations while RD vs. MWF, MD vs. MWF, and AxD vs. MWF showed significant negative correlations. However, in terms of effect size, the DTI indices exhibited weak-to-moderate correlations with MWF. This supports the notion that these DTI indices, and especially FA and RD, cannot serve as specific markers of myelin content (5-8), clearly indicating that any single MRI parameter alone cannot describe the temporal and spatial maturation and degeneration processes involved in senescence. This highlights the value of using multiple advanced and conventional quantitative MRI metrics that are specific and sensitive to distinct tissue features for clinical research (18-20).
Conclusions
On a uniquely large cohort of cognitively normal adults and using MWF and DTI mapping, we showed that brain maturation continues until middle age followed by a phase of rapid degeneration at older ages. Sexual dimorphism in all these parameters was not significant in most white matter regions studied. Finally, we observed weak associations between DTI indices and MWF, strongly indicating the potential of using these outcome measures in a multi-parametric approach to characterize age- and sex-related changes.Acknowledgements
This work was supported by the Intramural Research Program of the National Institute on Aging of the National Institutes of Health.References
1. Peters A. The effects of normal aging on myelin and nerve fibers: a review. Journal of neurocytology. 2002;31(8-9):581-93.
2. Tang Y, Nyengaard JR, Pakkenberg B, Gundersen HJ. Age-induced white matter changes in the human brain: a stereological investigation. Neurobiology of aging. 1997;18(6):609-15.
3. Laule C, Leung E, Lis DK, Traboulsee AL, Paty DW, MacKay AL, et al. Myelin water imaging in multiple sclerosis: quantitative correlations with histopathology. Multiple sclerosis (Houndmills, Basingstoke, England). 2006;12(6):747-53.
4. MacKay AL, Laule C. Magnetic Resonance of Myelin Water: An in vivo Marker for Myelin. Brain Plasticity. 2016;2(1):71-91.
5. Billiet T, Vandenbulcke M, Mädler B, Peeters R, Dhollander T, Zhang H, et al. Age-related microstructural differences quantified using myelin water imaging and advanced diffusion MRI. Neurobiology of aging. 2015;36(6):2107-21.
6. Arshad M, Stanley JA, Raz N. Adult age differences in subcortical myelin content are consistent with protracted myelination and unrelated to diffusion tensor imaging indices. NeuroImage. 2016;143:26-39.
7. Mädler B, Drabycz SA, Kolind SH, Whittall KP, MacKay AL. Is diffusion anisotropy an accurate monitor of myelination? Correlation of multicomponent T2 relaxation and diffusion tensor anisotropy in human brain. Magnetic resonance imaging. 2008;26(7):874-88.
8. Faizy TD, Thaler C, Broocks G, Flottmann F, Leischner H, Kniep H, et al. The Myelin Water Fraction Serves as a Marker for Age-Related Myelin Alterations in the Cerebral White Matter – A Multiparametric MRI Aging Study. Frontiers in Neuroscience. 2020;14(136).
9. Bouhrara M, Spencer RG. Incorporation of nonzero echo times in the SPGR and bSSFP signal models used in mcDESPOT. Magnetic resonance in medicine. 2015;74(5):1227-35.
10. Bouhrara M, Spencer RG. Improved determination of the myelin water fraction in human brain using magnetic resonance imaging through Bayesian analysis of mcDESPOT. NeuroImage. 2016;127:456-71.
11. Bouhrara M, Spencer RG. Rapid simultaneous high-resolution mapping of myelin water fraction and relaxation times in human brain using BMC-mcDESPOT. NeuroImage. 2017;147:800-11.
12. Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. NeuroImage. 2012;62(2):782-90.
13. Faizy TD, Kumar D, Broocks G, Thaler C, Flottmann F, Leischner H, et al. Age-Related Measurements of the Myelin Water Fraction derived from 3D multi-echo GRASE reflect Myelin Content of the Cerebral White Matter. Scientific Reports. 2018;8(1):14991.
14. Bouhrara M, Rejimon AC, Cortina LE, Khattar N, Bergeron CM, Ferrucci L, et al. Adult brain aging investigated using BMC-mcDESPOT based myelin water fraction imaging. Neurobiology of aging. 2020;85:131-9.
15. Cox SR, Ritchie SJ, Tucker-Drob EM, Liewald DC, Hagenaars SP, Davies G, et al. Ageing and brain white matter structure in 3,513 UK Biobank participants. Nature Communications. 2016;7(1):13629.
16. Fjell AM, Engvig A, Tamnes CK, Grydeland H, Walhovd KB, Westlye LT, et al. Life-Span Changes of the Human Brain White Matter: Diffusion Tensor Imaging (DTI) and Volumetry. Cerebral Cortex. 2009;20(9):2055-68.
17. Westlye LT, Walhovd KB, Dale AM, Bjørnerud A, Due-Tønnessen P, Engvig A, et al. Life-span changes of the human brain white matter: diffusion tensor imaging (DTI) and volumetry. Cerebral cortex (New York, NY : 1991). 2010;20(9):2055-68.
18. Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4(3):316-29.
19. Alexander AL, Hurley SA, Samsonov AA, Adluru N, Hosseinbor AP, Mossahebi P, et al. Characterization of Cerebral White Matter Properties Using Quantitative Magnetic Resonance Imaging Stains. Brain Connect. 2011;1(6):423-46.
20. Feldman HM, Yeatman JD, Lee ES, Barde LHF, Gaman-Bean S. Diffusion Tensor Imaging: A Review for Pediatric Researchers and Clinicians. J Dev Behav Pediatr. 2010;31(4):346-56.
Figures