1941
Testing the cognitive reserve hypothesis in the non-Western populations: evidence from a multicentric neuroimaging study in India1CSCR, University of Southern California, Los Angeles, CA, United States, 2Center for Mind/Brain Sciences (CIMEC),, University of Trento, Trento, Italy, 3Multimodal Brain Image Analysis Laboratory, National Institute of Mental Health and Neurosciences, Bangalore, India, 4Laboratory of Neuro Imaging, University of Southern California, Los Angeles, CA, United States, 5All India Institute of Medical Sciences, New Delhi, India, 6Davis School of Gerontology, University of Southern California, Los Angeles, CA, United States
Synopsis
Education offers neuroprotective effects against the progression of dementia. Limited information exists about such effects in non-Western populations, where formal education can be critically reduced. Here we examined dementia and education effects on brain morphometry in elderly healthy and mild cognitive impaired Indians. Morphometry revealed atrophy in areas typically related to MCI, enlarged lateral ventricles and reduced hippocampal volume. Education increased cortical thickness atrophy in the parahippocampal and temporal cortices (MCI group). This supports the cognitive reserve hypothesis, in which inter-individual differences in task processing is believed to allow some individuals to better cope with the neuropathology associated with dementia.
Introduction
Education has been found to be a modifiable risk factor decreasing the prevalence of Alzheimer's disease in Western populations. It is still unclear how education modulates the degree of cortical atrophy in mild cognitive impairment (MCI) and Alzheimer's disease (AD) subjects, particularly in non-Western populations, where formal education can be reduced. Here we utilized the Longitudinal Aging Study in India - Diagnostic Assessment of Dementia (LASI-DAD) [1,X] cohort to examine the relationship between cognitive status, education, and structural morphometry [1,X]. Unique to this cohort is the sizable number of uneducated elderly Indians.Methods
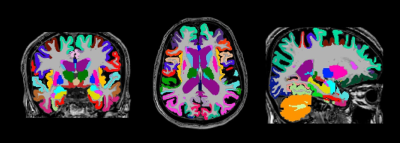
Structural 3T MRI was conducted on 55 healthy elderly controls and 75 persons with MCI at three clinical MRI centers in India. Average participant age was 67 (sd = 6) years of age, 35 participants (27% of sample) had received no formal education. Structural analysis consisted of 2x averaged 3D MP-RAGE scans, 3D FLAIR for pial segmentation, and 2D T2 high resolution image of the hippocampus for hippocampal segmentation using Freesurfer v6.0. Effects of MCI and education were examined with Bayesian general linear models controlling for age, gender, and intracranial volume on cortical ROIs consisting of gray matter volume, surface area, cortical thickness, and cortical thickness variability measures. The multicentric harmonized structural MRI protocol generated good quality fully automated brain segmentations (Figure 1, Figure 2).Results
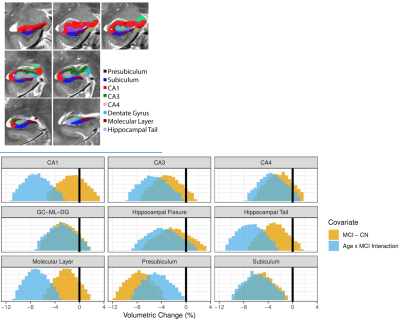
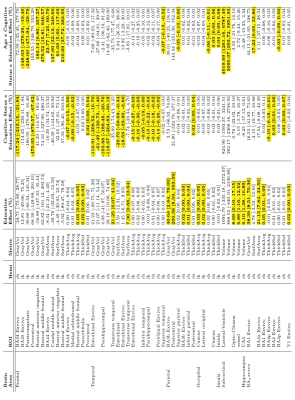
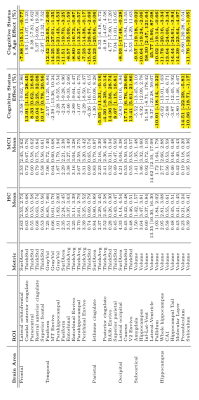
Education showed found to have a significant positive association with intracranial volume (Table 1). Education also showed a positive association with increased rostral middle frontal and lateral occipital cortical thickness. Confirming previous studies [9], education was also found to be positively associated with CA3 hippocampal subfield volume. Education and cognitive status interactions were found to be negatively associated with temporal cortical thickness, including the inferior temporal, parahippocampal, and superior temporal areas. In terms of MCI effects, our main results were consistent with previous studies. Lateral ventricles and hippocampus were found to be associated with MCI (Table 2). Specifically, mean lateral ventricle volume was found to be 13973 mm3 with a standard deviation of 6652 mm3 and was positively associated with the interaction between age and MCI [β = 3191 mm3, Cohen’s D = -0.96]. Whereas, mean hippocampus volume was 2850 mm3, standard deviation 368 mm3^3 and was negatively associated with the age-MCI interaction term [β = -191.3 mm3, Cohen’s D = -1.03]. Reduced cortical thickness for MCI was found in the posterior cingulate and isthmus cingulate. MCI participants were found to have reduced cortical thickness with age compared to healthy controls (cognitive status x age interaction) in the entorhinal, parahippocampal, and perirhinal cortices. Cortical thickness variability was significantly increased in MCI relative to HC, primarily in the frontal lobe. Differences in gray matter volume and surface area were found primarily in temporal areas of the brain, with the significant effect being driven by the cognitive status x age interaction. Specifically, age was found to significantly reduce gray matter volume or surface area, more in MCI relative to HC, in the parahippocampal, fusiform, isthmus cingulate, lateral occipital, and lateral orbitofrontal cortices. The hippocampus was segmented into 8 subfields and examined for associations with MCI (Figure 2). A significant reduction of subfield volume with increasing age in MCI compared to HC in CA1, hippocampal tail, molecular layer, presubiculum, and subiculum was found. Alongside the significant cognitive status x age interaction, the presubiculum and subiculum were also found to be significantly reduced in volume for MCI compared to HC. [AL1]Perhaps move this to methods as well.Discussion
Consistent with previous studies, we found that education was significantly correlated with increased intracranial volume [2]. The interaction between education, age, and cognitive status correlated with enlarged lateral ventricles, and the interaction between education and cognitive status was negatively associated with entorhinal and parahippocampal gray matter volume. This suggests that MCI subjects with increased education showed increased atrophy, lateral ventricle enlargement and cortical thinning. These results align with the cognitive reserve hypothesis, which maintains that subjects with increased cognitive reserve (e.g., increased education), can withstand greater neurodegeneration before displaying cognitive deficits [3, 4].We observed distributed cortical atrophy in MCI with respect to the healthy elderly controls, which led to decreased gray matter volume, surface area, and cortical thickness primarily in the temporal and parietal lobes. These findings are in good agreement with other aging studies, such as those that found decreased cortical thickness in entorhinal cortex, parahippocampal gyrus, posterior cingulate, isthmus of cingulate gyrus [5-8]. Along with cortical atrophy, in MCI we also found distributed subcortical gray matter and ventricular atrophy that is in good agreement with previous studies [9].Conclusions
Our findings support the cognitive reserve hypothesis in an Indian population of healthy elderly and MCI subjects. Specifically, we found greater cortical atrophy in educated elderly that have MCI compared to those with less education. This supports the idea that even reduced formal education allows for greater resistance to the cognitive effects associated with the neurodegenerative effects of MCI and Alzheimer's disease.Acknowledgements
No acknowledgement found.References
1. Lee, J., Khobragade, P. Y., Banerjee, J., Chien, S., Angrisani, M., Perianayagam, A., Bloom, D. E., & Dey, A. B. (2020). Design and Methodology of the Longitudinal Aging Study in India‐Diagnostic Assessment of Dementia ( LASI‐DAD ). Journal of the American Geriatrics Society, 68(S3). https://doi.org/10.1111/jgs.16737
2. Foubert-Samier, A., Catheline, G., Amieva, H., Dilharreguy, B., Helmer, C., Allard, M., & Dartigues, J.-F. (2012). Education, occupation, leisure activities, and brain reserve: a population-based study. Neurobiology of Aging, 33(2), 423.e15-423.e25. https://doi.org/10.1016/j.neurobiolaging.2010.09.023
3. Mungas, D., Gavett, B., Fletcher, E., Farias, S. T., DeCarli, C., & Reed, B. (2018). Education amplifies brain atrophy effect on cognitive decline: implications for cognitive reserve. Neurobiology of Aging, 68, 142–150. https://doi.org/10.1016/j.neurobiolaging.2018.04.002
4. Liu, Y., Julkunen, V., Paajanen, T., Westman, E., Wahlund, L.-O., Aitken, A., Sobow, T., Mecocci, P., Tsolaki, M., Vellas, B., Muehlboeck, S., Spenger, C., Lovestone, S., Simmons, A., & Soininen, H. (2012). Education increases reserve against Alzheimer’s disease—evidence from structural MRI analysis. Neuroradiology, 54(9), 929–938. https://doi.org/10.1007/s00234-012-1005-0
5. Jiang, L., Cao, X., Jiang, J., Li, T., Wang, J., Yang, Z., & Li, C. (2019). Atrophy of hippocampal subfield CA2/3 in healthy elderly men is related to educational attainment. Neurobiology of Aging, 80, 21–28. https://doi.org/10.1016/j.neurobiolaging.2019.03.019
6. Convit, A., De Leon, M. J., Tarshish, C., De Santi, S., Tsui, W., Rusinek, H., & George, A. (1997). Specific Hippocampal Volume Reductions in Individuals at Risk for Alzheimer’s Disease. Neurobiology of Aging, 18(2), 131–138. https://doi.org/10.1016/s0197-4580(97)00001-8
7. Korf, E. S. C., Wahlund, L.-O., Visser, P. J., & Scheltens, P. (2004). Medial temporal lobe atrophy on MRI predicts dementia in patients with mild cognitive impairment. Neurology, 63(1), 94–100. https://doi.org/10.1212/01.wnl.0000133114.92694.93
8. Platero, C., López, M. E., Carmen Tobar, M. del, Yus, M., & Maestu, F. (2018). Discriminating Alzheimer’s disease progression using a new hippocampal marker from T1-weighted MRI: The local surface roughness. Human Brain Mapping, 40(5), 1666–1676. https://doi.org/10.1002/hbm.24478
9. Li, Y.-D., Dong, H.-B., Xie, G.-M., & Zhang, L. (2013). Discriminative Analysis of Mild Alzheimer’s Disease and Normal Aging Using Volume of Hippocampal Subfields and Hippocampal Mean Diffusivity. American Journal of Alzheimer’s Disease & Other Dementiasr, 28(6), 627–633. https://doi.org/10.1177/1533317513494452
Figures