1939
1-Norm for quantifying the degree of brain tissue mechanical inhomogeneity due to neurodegenerative disease1Department of Mechanical and Industrial Engineering, University of Illinois at Chicago, Chicago, IL, United States, 2Richard and Loan Hill Department of Bioengineering, University of Illinois at Chicago, Chicago, IL, United States, 3Charite Universitatsmedizin, Berlin, Germany
Synopsis
In prior investigations, we have established 1-Norm as a quantitative measure of degree of inhomogeneity of excised biological tissues. Here, we apply 1-Norm to study effect of Alzheimer’s disease on mechanical inhomogeneity of brain. MRE was performed on excised brains of 3 control mice & 3 mice with AD(5xFAD). 1-Norm revealed increase in mechanical inhomogeneity of brain tissue due to AD. We speculate this increase in mechanical inhomogeneity of brains with AD may be due to amyloid plaque deposition, synaptic degeneration, neuronal loss & loss of white matter tracts. We aim to establish 1-Norm as a biomarker to detect AD in humans.
Introduction
Alzheimer’s disease (AD) leads to a reduction in the stiffness of the brain1. In the current work, we use Magnetic Resonance Elastography (MRE), to measure the magnitude of scattering of shear waves in both healthy murine brain samples and in brains of an AD mouse model (5xFAD). We use a waveform analysis technique called 1-Norm2 to quantify the magnitude of scattering of shear waves due to mechanical property inhomogeneity in each of the brain samples.Hypothesis
Our hypothesis is that AD leads to a change in the mechanical inhomogeneity of the brain tissue due to degeneration of white matter fiber tracts (axonal damage3) and due to amyloid plaque deposition4. We propose to test this hypothesis through analysis of contours of shear wave displacement generated using MRE as described below.Method
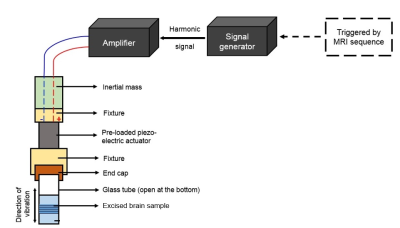
6 Brain samples (3 from control mice and 3 from 5xFAD AD female mice) were excised after euthanizing individual mice at 10 month age. Each brain sample was then carefully placed in a 10 mm diameter borosilicate glass tube, whose one end was connected to a piezoelectric actuator as shown in fig. 1. This set up was then introduced into the vertical bore of a Low-field (0.5 T) MRI scanner (Pure Devices, Würzburg-Rimpar, Germany). Geometrically focussed shear waves5 were introduced into each brain sample with MRE experimental parameters as enlisted in Table 1.Data Processing
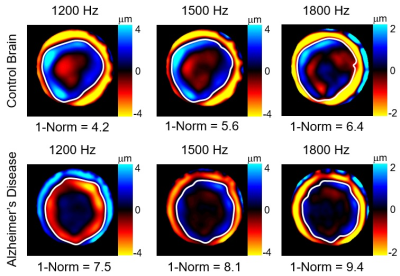
Image reconstruction was performed on the phase images at each mechanical frequency, by first applying Flynn Phase-unwrapping algorithm. Subsequently, a band-pass filter was used to eliminate the compression wave component and noise in each of the phase images. Complex displacement wave images were obtained by choosing the first harmonic after Fourier transformation along the time-resolved and filtered phase images. Contours of equal wave displacement (for example, zero shear wave displacement) were identified in the complex wave image (real part) and delineated using the contour command in Matlab. The 1-Norm wave form analysis technique was applied to each delineated contour as previously described2 and as shown in fig. 2.Results
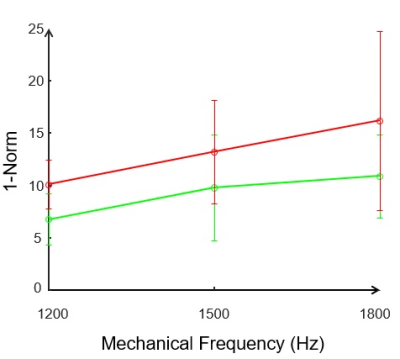
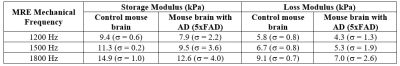
1-Norm quantifies the magnitude of scattering of shear waves due to mechanical property inhomogeneity2. Color-encoded images of shear wave displacements and the corresponding delineated wavefront (white color) for 2 brain samples (Control and AD) are shown in fig. 2. Neurodegenerative disease leads to an increase in the magnitude of scattering of shear waves at each mechanical frequency as is evident from figure 3, in which mean values of 1-Norm for both control brain samples and brain samples of AD mouse model 5xFAD, along with standard deviation are shown. This increase in shear wave scattering must be due to an increase in degree of mechanical property inhomogeneity of the AD mouse model brain2. In addition, the scattering becomes more pronounced at higher mechanical frequencies, which is in line with observations in a previous study2. A two-way analysis of variance (ANOVA) showed a statistical difference (fig. 3) between 1-Norm values of three control brain samples and the three brain samples of AD mouse model with p-value < 0.0001. In addition, the storage and loss moduli for each of the brain samples were determined and it was found that the brains of mice with AD (5xFAD) have reduced stiffness in comparison to control mice (Table 2), which is consistent with research findings of other research groups1. An ANOVA assessment of the storage and loss moduli for three control and three AD brain samples gave statistical difference with p < 0.0001.Discussion
Our results (figs. 2 and 3) suggest that neurodegenerative disease leads to increase in mechanical property inhomogeneity of brain tissue. The 3 AD mice (5xFAD) were 10 months old at the time of sacrifice (MRE experimentation) and had developed severe amyloid plaque deposition6 throughout the hippocampus and cortex region. Synaptic degeneration4 and neuron loss4 have also been observed in mice belonging to this model starting from 6 months of age. Additionally, a prior DTI-MRI3 based clinical investigation on human subjects revealed an increased axial diffusivity in the brains with AD, which the researchers had attributed to axonal damage (which led to an increase in extra axonal space). We therefore speculate that these 4 neurological conditions led to an observable increase in the degree of inhomogeneity of the brain tissue for the three samples of 10-month old female AD mice (5xFAD) used in our study. Further studies are needed to understand the microstructural changes that lead to an increase in the degree of inhomogeneity of the brain tissue due to AD.Conclusion
Mechanical property inhomogeneity assessment based on the 1-Norm technique revealed an increase in the degree of inhomogeneity of the brain tissue in neurodegenerative disease due to the presence of pathological conditions. The 1-Norm technique represents an alternative MRE technique that does not rely on ill-posed wave field inversion techniques and has potential to serve as a biomarker for neurodegenerative disease diagnosis and staging. Further studies including histopathology are needed to shed light on the microscale causes of mechanical property inhomogenization in neurodegenerative disease. Our preliminary investigation is in line with the findings of other research groups, namely, that AD leads to a reduction in brain tissue stiffness1.Acknowledgements
The financial support of NSF Grant 1852691 is kindly acknowledged.References
1. Murphy, M. C. et al. Decreased brain stiffness in Alzheimer’s disease determined by magnetic resonance elastography. J. Magn. Reson. Imaging 34, 494–498 (2011).
2. Palnitkar, H. et al. An investigation into the relationship between inhomogeneity and wave shapes in phantoms and ex vivo skeletal muscle using Magnetic Resonance Elastography and finite element analysis. J. Mech. Behav. Biomed. Mater. 98, 108–120 (2019).
3. Molinuevo, J. L. et al. White matter changes in preclinical Alzheimer’s disease: A magnetic resonance imaging-diffusion tensor imaging study on cognitively normal older people with positive amyloid β protein 42 levels. Neurobiol. Aging 35, 2671–2680 (2014).
4. Oakley, H. et al. Intraneuronal β-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: Potential factors in amyloid plaque formation. J. Neurosci. 26, 10129–10140 (2006).
5. Yasar, T. K., Royston, T. J. & Magin, R. L. Wideband MR elastography for viscoelasticity model identification. Magn. Reson. Med. 70, 479–489 (2013).
6. Mattana, S., Caponi, S., Tamagnini, F., Fioretto, D. & Palombo, F. Viscoelasticity of amyloid plaques in transgenic mouse brain studied by Brillouin microspectroscopy and correlative Raman analysis. J. Innov. Opt. Health Sci. 10, (2017).
Figures