1934
Thalamic nuclei changes in prodromal and clinical Alzheimer’s disease
Adam S Bernstein1, Steve Z Rapcsak2, Michael Hornberger3, and Manojkumar Saranathan4
1College of Medicine, University of Arizona, Tucson, AZ, United States, 2Department of Neurology, University of Arizona, Tucson, AZ, United States, 3Department of Medicine, University of East Anglia, Norwich, United Kingdom, 4Department of Medical Imaging, University of Arizona, Tucson, AZ, United States
1College of Medicine, University of Arizona, Tucson, AZ, United States, 2Department of Neurology, University of Arizona, Tucson, AZ, United States, 3Department of Medicine, University of East Anglia, Norwich, United Kingdom, 4Department of Medical Imaging, University of Arizona, Tucson, AZ, United States
Synopsis
Using a novel multi-atlas segmentation technique, we studied the atrophy of thalamic nuclei as a function of disease progression in Alzheimer’s disease. We found statistically significant atrophy of the anteroventral, centromedian, and mediodorsal nuclei, which are part of the limbic system and known to play a known role in memory and cognitive function. We also found atrophy of the medial geniculate nucleus and the pulvinar nucleus. The degree of atrophy increases from early MCI to full AD. These findings suggest that a larger network of brain structures are affected in Alzheimer’s disease, which together lead to the clinical presentation.
Introduction
Increasing evidence suggests that the thalamus, and particularly the limbic thalamic nuclei may play a central role in the pathogenesis of Alzheimer’s disease (AD).1 While whole thalamic volume atrophy and shape changes have been reported, only limited research has been conducted to investigate atrophy in individual thalamic nuclei in AD, likely due to the paucity of accurate high resolution thalamic nuclei segmentation methods. This work utilizes a novel multi-atlas segmentation technique to parcellate the thalamus into individual nuclei and explore changes in thalamic nuclear volumes across the AD continuum (i.e. prodromal and clinical) using data from the ADNI database. We hypothesize that there will be significant atrophy of specific thalamic nuclei, particularly those associated with memory and the Papez circuit, including the anteroventral (AV) nucleus, and the mediodorsal (MD) nucleus, with increasingly severity leading up to AD.Methods
3T T1-weighted Magnetization Prepared RApid Gradient Echo (MPRAGE) data from the ADNI database were used in this study. Healthy controls (HC, n=119), early mild cognitive impairment (EMCI, n=208), late mild cognitive impairment (LMCI, n=116), and AD subjects (n=91) were selected. The MPRAGE data were parcellated using a variant of the recently proposed THalamus Optimized Multi-Atlas Segmentation (THOMAS) technique of Su et al2 to determine volumes of 11 individual thalamic nuclei on each side. For this work, THOMAS was modified to use majority voting for label fusion (as opposed to joint fusion) to enable the use of standard CSF-nulled T1-weighted MPRAGE data as input as opposed to white-matter-nulled (WMn) MPRAGE of Su et al.The proposed segmentation pipeline is depicted in Figure 1. Briefly, a WMn template image was generated by mutual registration and averaging of 20 WMn MPRAGE priors, each with manually labelled thalamic nuclei. The template was first rigidly registered to the subject image using ANTs.3 Following rigid registration, both the template and the subject image were cropped to improve registration speed (by 10X) and accuracy. The cropped template image was then nonlinearly registered to the subject image, using ANTs (SyN diffeomorphic). The nonlinear warps from each of the 20 priors to the template is pre-computed and available. Using a composite of prior-template and template-subject warps, manual nuclei labels from all 20 priors were warped into subject space and then combined using a majority voting based label fusion scheme to produce subject-specific thalamic nuclei labels.
Thalamic nuclear volumes across the different clinical subgroups were compared using ANCOVA after correcting for intracranial volume and age. In addition to volume comparisons across groups, correlations of limbic nuclei volumes with neurocognitive testing scores, clinical disease metrics including the clinical dementia rating (CDR) and the Alzheimer’s disease assessment scale with 13 elements (ADAS13), and phosphorylated tau protein (P-Tau) and beta amyloid (Aβ) levels were performed.
Results
Thalamic nuclei segmentation labels from the modified THOMAS method overlaid on MPRAGE data in axial (top panel) and coronal (bottom panel) are shown in Figure 2 for a patient with AD (enlarged ventricles). Small nuclei such as anteroventral (AV), centromedian (CM), and the habenula (Hb) are clearly delineated.Results of the volume analysis are shown in Figure 3. The volumes of the AV, Pul, MGN, CM, and MD nuclei were significantly smaller in subjects with AD when compared to HC subjects. Further, the AV, Pul, MGN, and MD nulcei were significantly smaller in subjects with LMCI when compared to HC subjects. No significant thalamic changes were found between HC subjects and EMCI patients. Cohen’s d, a metric that measures effect size, is plotted in Figure 4 for all nuclei that demonstrated significant volume differences. There is a progressive increase in effect size with increasing disease severity when volumes are compared to those of the HC group. The correlation analysis, summarized in Table 1, demonstrates mild to moderate correlations that are statistically significant for the AV and MD nuclei with neurocognitive scores (MoCA and RAVLT) and clinical assessments (CDR and ADAS13). Of all the thalamic nuclei, the AV nuclei volumes were the only ones that demonstrated a significant correlation to P-tau levels. The MD showed a significant correlation with Aβ.
Conclusions and Discussion
While previous work has shown whole thalamic volume atrophy and thalamic nuclei in AD4, this is the first work to systematically examine thalamic nuclei atrophy across prodromal and clinical AD using a fast and accurate thalamic nuclei segmentation method. This preliminary work highlights nuclei-specific atrophy within the thalamus in subjects with LCMI and AD. This is consistent with the hypothesis that the memory and cognitive changes in AD are mediated by damage to a large, integrated neural network that extends beyond the medial temporal lobes, (notably the hippocampus and related structures) which have been the traditional focus of attention in AD research.Acknowledgements
Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defenseaward number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech;
BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research &
Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of
Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated
by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
References
- Aggleton JP. Multiple anatomical systems embedded within the primate medial temporal lobe: Implications for hippocampal function. Neurosci Biobehav Rev.; 36:1579-1596 (2012)
- Su JH, Thomas FT, Kasoff WS, et al. Thalamus Optimized Multi Atlas Segmentation (THOMAS): fast, fully automated segmentation of thalamic nuclei from structural MRI. Neuroimage; 194:272-282 (2019).
- Avants BB, Tustison NJ, Stauffer M, Song G, Wu B, Gee JC. The Insight ToolKit image registration framework. Front Neuroinform. 2014;8(APR). doi:10.3389/fninf.2014.00044
- Iglesias JE et al. A probabilistic atlas of the human thalamic nuclei combining ex vivo MRI and histology. Neuroimage; 183:314-326 (2018)
Figures
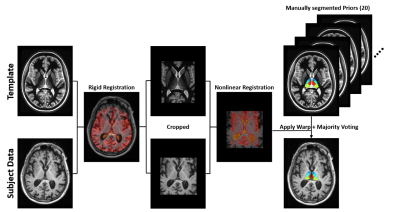
Figure 1. Multi-atlas segmentation scheme for thalamic nuclei segmentation. The multi-atlas consists of 20 manually segmented WMn MPRAGE data which are warped to subject space and label fused using a majority voting scheme. A WMn template is used as an intermediate step to improve robustness and cropping is performed to improve speed and accuracy.
Figure 2. Thalamic nuclei segmentation labels from the modified THOMAS method
overlaid on MPRAGE data in axial (top panel) and coronal (bottom panel) for a patient with AD (enlarged ventricles). Note visualization of small structures such as anteroventral (AV), centromedian (CM) and habenula (Hb).
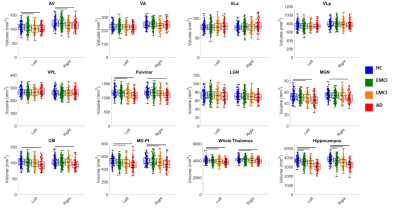
Figure 3. Thalamic nuclei volumes plotted as box and whisker plots
compared across four groups- Healthy controls (HC),
early mild cognitive impairment (EMCI), late mild cognitive
impairment (LMCI), and Alzheimer’s disease (AD). Each
subject’s nuclei volume is plotted as a filled circle on the plot. The
black “x” on each box and whisker plot denotes the mean volume for each
group. Statistically significant differences (p < 0.05) in pairwise
comparisons are shown as black bars above the box
and whisker plots.
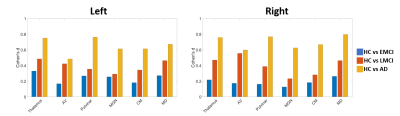
Figure 4. Effect sizes plotted as Cohen’s d computed from pairwise
comparisons of healthy controls (HC) with early mild cognitive
impairment (EMCI), late mild cognitive impairment (LMCI), and
Alzheimer’s disease (AD), for the whole thalamus, hippocampus, and
individual thalamic nuclei. Note progressive increase in d across the disease process.

Table 1. Pearson's correlation coefficient (β) and associated p-values
of the anteroventral (AV) and the mediodorsal (MD) nuclei with select
neurocognitive scores, clinical assessments, and biomarker levels.