1931
Impact of Dementia with Lewy Bodies on Brain Biomechanical Properties1Department of Radiology, Mayo Clinic, Rochester, MN, United States, 2Department of Physiology and Biomedical Engineering, Mayo Clinic College of Medicine, Rochester, MN, United States, 3Department of Neurology, Mayo Clinic, Rochester, MN, United States, 4Division of Pulmonary and Critical Care Medicine, Mayo Center for Sleep Medicine, Mayo Clinic, Rochester, MN, United States
Synopsis
Dementia with Lewy Bodies (DLB) is the second most common neurodegenerative dementia in older people after Alzheimer’s, accounting for 10-15% of all dementia cases. In this study we used Magnetic Resonance Elastography (MRE) to assess the feasibility of using the changes in brain mechanical properties as potential biomarkers.
Target Audience:
MR Elastography researchers, radiologists and neurologists.Introduction:
Dementia with Lewy bodies(DLB) manifests as progressive cognitive decline, typically in conjunction with REM sleep behavior disorder, cognitive fluctuations, Parkinsonism, and visual hallucinations1. Pathologically, DLB is characterized by progressive aggregation of the synaptic protein alpha-synuclein(α-syn) as Lewy bodies within neurons of the brainstem, limbic and neocortical regions2. Due to phenotypic overlap with Parkinsonism and Alzheimer’s disease(AD), DLB remains under detected and often misdiagnosed3. MRE is an emerging in vivo technique to quantitatively measure the biomechanical properties of tissues, with demonstrated sensitivity to various neurodegenerative processes4. This study is designed to explore the changes in viscoelastic parameters of brain associated with DLB with two main aims. First, we used a sufficiently powered sample to confirm previously reported preliminary results using an established direct inversion(DI)-based regional pipeline5. Second, we performed exploratory analyses using a neural network inversion(NNI) to allow stable mechanical property estimation near edges, enabling more repeatable measurements and robust calculations of both stiffness and damping ratio6.Methods:
Study Participants: This study was approved by the Mayo Clinic Institutional Review Board and written informed consent was obtained from the volunteers and/or their proxies before performing the experiments. A total of 57 participants were recruited, consisting of 44 cognitively unimpaired controls(CU) with age 56-87 years from the Mayo Clinic Study of Aging, and 13 patients with probable DLB with age 56-75 years from the Mayo Clinic Alzheimer’s disease Research Center. Patients with probable DLB were diagnosed according to 4th Consortium Criteria for DLB1.MRE experiments: Participants were scanned on 3T GE scanners (GE, Waukesha, WI) with an 8-channel GE receive-only head coil. MRE data were collected with a single-shot spin-echo EPI pulse sequence using 60Hz vibration and image resolution of 3mm isotropic, as previously described.7 T1-weighted images acquired in the same exam were used for brain segmentation and to define regions of interest using an in-house template and atlas by unified segmentation in SPM.8,9
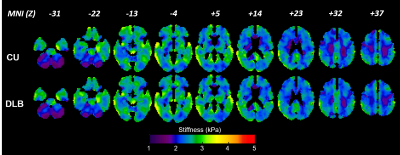
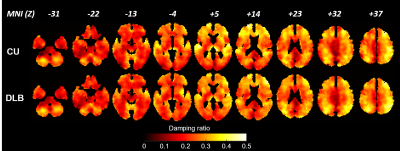
Image processing: MRE data were processed using the DI-based pipeline described previously5 for measuring lobar brain stiffness. The median stiffness in 8 regions was calculated for each subject, including cerebrum, frontal lobes, occipital lobes, parietal lobes, temporal lobes, deep gray matter/white matter, cerebellum and the brain stem. Additionally, stiffness and damping ratio maps were computed for each participant using a previously trained neural network inversion10. Mean mechanical property for NNI-based stiffness and damping ratio were calculated for each of 34 regions using an in-house modified version of the automated anatomical labeling(AAL) atlas. For display, each participant’s mechanical property maps were spatially normalized to an in-house template9, and the mean mechanical property maps were computed for each group.
Statistical Analysis: For both DI- and NNI-based stiffness, we tested the hypothesis that DLB and CU participants had significantly different stiffness in each region by two-sample t-test while fixing the effects of age and sex. A false discovery rate (FDR) corrected Q-value was computed for each region using Storey’s method. Max(P,Q)<0.05 was considered significant. Similar analysis was performed on NNI-estimated damping ratio for each region.
Results and Discussion:
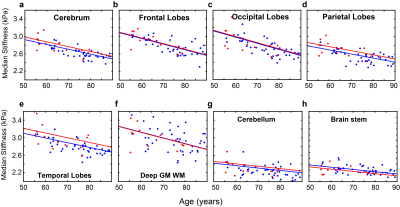
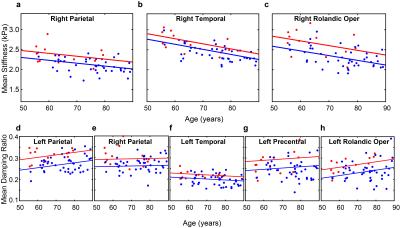
The DI-based results for stiffness over 8 lobar regions are summarized in Figure 1. After FDR correction, no significant differences were detected. The stiffness of temporal lobes increased and exhibited the largest effect due to DLB (uncorrected P=0.032). This may be related to higher Lewy body density in the inferior temporal cortex in the DLB group associated with cognitive fluctuations and visual hallucinations10. Stiffness significantly decreased with age in all regions (P<0.0001 for cerebrum, frontal, occipital, parietal and temporal lobes, P=0.0028 for deep GM/WM, P=0.0021 for cerebellum and P=0.0002 for brain stem). These results are consistent with our aging study of the brain11. Average stiffness and damping ratio maps estimated by NNI are shown in Figures 2 and 3, respectively. Of the 34 gray matter regions, statistically significant changes (max(P,Q)<0.05) are observed in 3 regions for stiffness and in 5 regions for damping ratio using NNI-based mechanical property maps(Figure 4). Statistically significant increase in stiffness is observed in DLB patients in the right parietal, temporal and Rolandic operculum with a maximum change 0.36 kPa. Statistically significant increase in damping ratio is observed in DLB patients in the left parietal, right parietal, left temporal, left precentral and left Rolandic operculum with a maximum change 0.039. Viscoelastic changes observed in some of these neocortical areas may relate to the accumulation of Lewy bodies, reflecting associated inflammation or dysregulation of neuronal cytoskeleton. This NNI was previously shown to be more resistant to noise- and edge-related bias6, and this study suggests that this type of inversion may provide improved sensitivity to pathological processes compared to a more traditional direct method.Conclusion:
Using previously established, DI-based regional measurements, we did not observe significant changes in brain stiffness due to probable DLB. However, in an analysis using NNI, small but significant increases in stiffness and damping ratio were observed in neocortical gray matter regions, mostly in the temporal and parietal lobes. Given that half or more of DLB patients may also have amyloid or tau pathology, which can also impact the brain’s mechanical properties, further investigation is needed to assess the differential effect of these various pathologies.Acknowledgements
This work is supported by grants from the NIH, EB027064, EB001981, U01 NS100620 and P50 AG062677.References
1. McKeith, I.G., et al. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology 89, 88-100 (2017).
2. Ferman, T.J., et al. The limbic and neocortical contribution of α-synuclein, tau, and amyloid β to disease duration in dementia with Lewy bodies. Alzheimer's & dementia : the journal of the Alzheimer's Association 14, 330-339 (2018).
3. Chin, K.S., Teodorczuk, A. & Watson, R. Dementia with Lewy bodies: Challenges in the diagnosis and management. The Australian and New Zealand journal of psychiatry 53, 291-303 (2019).
4. Murphy, M.C., Huston, J., 3rd & Ehman, R.L. MR elastography of the brain and its application in neurological diseases. NeuroImage 187, 176-183 (2019).
5. Oliphant, T.E., Manduca, A., Ehman, R.L. & Greenleaf, J.F. Complex-valued stiffness reconstruction for magnetic resonance elastography by algebraic inversion of the differential equation. Magnetic resonance in medicine 45, 299-310 (2001).
6. Murphy, M.C., et al. Identification of Normal Pressure Hydrocephalus by Disease-Specific Patterns of Brain Stiffness and Damping Ratio. Investigative radiology 55, 200-208 (2020).
7.Murphy, M.C., et al. Measuring the characteristic topography of brain stiffness with magnetic resonance elastography. PloS one 8, e81668 (2013).
8. Ashburner, J. & Friston, K.J. Unified segmentation. NeuroImage 26, 839-851 (2005).
9. Vemuri, P., et al. Alzheimer's disease diagnosis in individual subjects using structural MR images: validation studies. NeuroImage 39, 1186-1197 (2008).
10. Harding, A.J., Broe, G.A. & Halliday, G.M. Visual hallucinations in Lewy body disease relate to Lewy bodies in the temporal lobe. Brain : a journal of neurology 125, 391-403 (2002).
11. Arani, A., et al. Measuring the effects of aging and sex on regional brain stiffness with MR elastography in healthy older adults. NeuroImage 111, 59-64 (2015).
Figures