1930
Application of SVS-PRESS, MEGA-PRESS, and pCASL to Evaluate Treatment Effect of Kami Guibi-tang in Patients with Mild Cognitive Impairment1Radiology, Kyung Hee University Hospital at Gangdong, Seoul, Korea, Republic of, 2Stroke and Neurological Disorders Center, Kyung Hee University Hospital at Gangdong, Seoul, Korea, Republic of, 3Radiology, The Johns Hopkins University School of Medicine, Baltimore, MD, United States
Synopsis
This study aims to evaluate the effectiveness of herb medicine in the treatment of mild cognitive impairment (MCI) using SVS PRESS MRS, MEGA-PRESS, and pCASL MRI. We randomly allocated a total of 30 MCI patients to an herb medicine or a placebo group and performed MRI scans before and after 24 weeks of treatment. We analyzed NAA/Cr, GABA/Cr, and cerebral blood flow (CBF) using repeated-measure analysis of variance (ANOVA). We found that the CBF measures were the most sensitive markers to evaluate the effect of the herb medicine on MCI.
Background
Mild cognitive impairment (MCI) is the stage between the expected cognitive decline of normal aging and the more serious decline of dementia. Amnestic MCI (aMCI) is a condition in which people experience memory problems more severe than normal for their age and education, but not enough to affect daily life. Kami Guibi-tang (KGT) is one of the famous herbal medicines used in Asia to treat amnesia, insomnia, loss of appetite, or depression. Previous studies have suggested that KGT could be beneficial for the cognitive functions of AD patients (1). However, only a few clinical trials have been published and the effect of KGT on MCI has not been investigated yet using MRI measures. Nevertheless, there is no proven treatment for MCI to date (2). In this trial, we explored the potential use of KGT as a therapeutic agent for MCI patients.Purpose
MRI measures may be a good tool to evaluate the impact of KTG on MCI patients. Therefore, this study aimed to evaluate the therapeutic effectiveness of a 24-weeks treatment with KGT granules using measurements of brain metabolite and neurotransmitter levels and CBF using a 3T MRI system.Methods
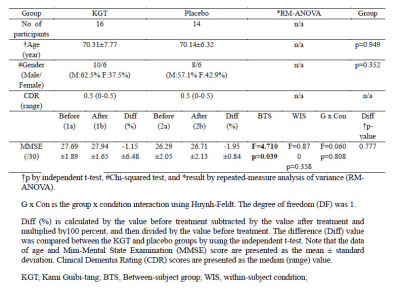
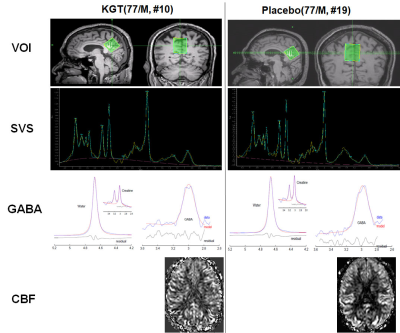
Participants: The institutional review board approved this cross-sectional prospective study. Table 1 summarizes the demographic characteristics and the results of the neuropsychological tests.MRI acquisition: The participants underwent brain MRIs at baseline and after 24 weeks of medication to obtain structural 3D T1-weighted (3D T1W) image, single-voxel proton magnetic resonance spectroscopy (1H-MRS, SVS-PRESS) with TE=35 ms PRESS for quantifying NAA, Cr, Cho, and glutamate complex (Glx), MEscher-GArwood (MEGA) Point-RESolved Spectroscopy (PRESS) for quantifying gamma-aminobutyric acid (GABA), and pseudo-continuous arterial spin labeling (pCASL) perfusion images with a 32-channel sensitivity encoding (SENSE) coil using a 3.0 Tesla MRI system (Ingenia, Philips Medical System, Best, The Netherlands). The cubic voxel size of 30 × 30 × 30 mm3 was placed at the precuneus and in the posterior cingulate areas of the brain for both 1H-MRS and MEGA-PRESS. Figure 1 depicts the representative volume-of-interest (VOI) for the proton single-voxel spectroscopy (SVS) and GABA MEGA-PRESS. For pCASL, we used label duration = 1650 ms, post-label delay = 1600 ms, label distance = 90 mm, and 35 pairs of labeled-control with two-pulse background suppression.
Post-Processing of MRI data: we processed pCASL images using SPM12 and a local Matlab code (3), PRESS SVS data using the 7.0.1 version of the MRSpectroView software (IntelliSpace Portal, Philips Medical Systems, Best, The Netherlands), and MEGA PRESS data using the 140709 version of the Gannet software package provided by the Johns Hopkins University (4).
Statistical analyses: CBF maps were analyzed by using both voxel-based and region-of-interest (ROI)-based methods. For the voxel-based CBF analysis, we performed 2x2 flexible factorial analysis of CBF maps to define the ROI areas. For the continuous variables such as MMSE scores, GABA+/Cr, Cho/Cr, NAA/Cr, mI/Cr, Glx/Cr, and ROI-based CBF, we used repeated-measure analysis of variance (RM-ANOVA).
Results
For MMSE scores, RM-ANOVA tests showed significantly difference between the KGT and placebo groups (F = 4.71, p = 0.039), not significantly difference between the before and after treatment conditions (F = 0.870, p = 0.358), and no group x condition interactions.Figure 1 shows the representative PRESS SVS spectra and MEGA PRESS spectra obtained from the VOI of the 77-year-old males in the KGT (10th participant) and placebo (19th participant) groups. For metabolite values and GABA, the GABA+/Cr values were significantly different between the KGT and placebo groups (F = 5.27, p = 0.029), not significantly different between the before and after treatment conditions, and not group x condition interactions. The metabolite values for Cho/Cr, NAA/Cr, mI/Cr, and Glx/Cr were not significantly different between the two groups, not group x condition interactions. The Glx/Cr values showed a difference trend between the two groups (F = 4.19, p = 0.050) and the group x condition interactions (F = 4.21, p = 0.050). The difference (Diff [%]) in the Glx/Cr values between the before and after treatment conditions were significantly different between the KGT and placebo groups (p = 0.042).
Figure 1 shows the representative normalized CBF maps obtained from the 77-year-old males in the KGT (10th participant) and placebo (19th participant) groups. VBM analysis showed that the CBF values were significantly different between the KGT and placebo groups and significantly different between before and after treatment conditions, but no group x condition interactions. ROI-based analyses showed that CBFs were significantly different between the two treatment conditions, but were not group x condition interactions for all the ROIs.
Conclusion
The CBF measure was the sensitive marker to evaluate the treatment effect of KGT on MCI participants. We found that the treatment affected the temporal lobe, including the hippocampus and the fusiform gyrus which are associated with memory. On the other hand, we measured almost no significant changes in brain metabolites which may be related to not enough dosage of KGT to improve the cognitive values. Therefore, further studies should be performed with a relatively larger population and increased dosage.Acknowledgements
The research was supported by the grant of the Convergence of Conventional Medicine and Traditional Korean Medicine R&D program funded by the Ministry of Health & Welfare through the Korea Health Industry Development Institute (KHIDI) (HI16C2352, GHJ) and by the National Research Foundation of Korea (NRF) grants funded by the Korean government (MEST) (No. 2020R1A2C1004749, GHJ).References
1)Watari H, et al. Evid Based Complement Alternat Med 2019; 2019: 4086749.
2)Tricco AC, et al. CMAJ 2013; 185: 1393-1401.
3)Wang Z, et al. Magn Reson Imaging 2008; 26: 261-269.
4)Edden RA, et al. J Magn Reson Imaging 2014; 40: 1445-1452.