1919
A neuroimaging study of the effects of early versus late anti-inflammatory treatment in the TgF344-AD rat model of Alzheimer’s disease
Caitlin F Fowler1,2, Dan Madularu3, Gabriel A Devenyi4,5, John Breitner5,6, and Jamie Near1,4,5
1Biological and Biomedical Engineering, McGill University, Montreal, QC, Canada, 2Cerebral Imaging Centre, Douglas Hospital Research Centre, Verdun, QC, Canada, 3Center for Translational Neuroimaging, Northeastern University, Boston, MA, United States, 4Cerebral Imaging Centre, Douglas Hospital Research Institute, Verdun, QC, Canada, 5Psychiatry, McGill University, Montreal, QC, Canada, 6Division of Human Neurosciences, Douglas Hospital Research Centre, Verdun, QC, Canada
1Biological and Biomedical Engineering, McGill University, Montreal, QC, Canada, 2Cerebral Imaging Centre, Douglas Hospital Research Centre, Verdun, QC, Canada, 3Center for Translational Neuroimaging, Northeastern University, Boston, MA, United States, 4Cerebral Imaging Centre, Douglas Hospital Research Institute, Verdun, QC, Canada, 5Psychiatry, McGill University, Montreal, QC, Canada, 6Division of Human Neurosciences, Douglas Hospital Research Centre, Verdun, QC, Canada
Synopsis
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder with no effective treatments or known biomarkers for definitive diagnosis, substantiating the need for early detection and intervention. This project employs Magnetic Resonance Spectroscopy to quantify neurochemical changes in the TgF344-AD rat model of AD in response to early versus late administration of a common non-steroidal anti-inflammatory drug, specifically addressing the critical question of treatment timing. Preliminary results suggest the TgF344-AD rat recapitulates most neurochemical features of human AD and that early treatment is more effective than late treatment at mitigating disease-related neurochemical changes.
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder with no effective treatments or known biomarkers for definitive diagnosis. Brain pathology is known to appear decades prior to clinical symptoms, substantiating the need for early detection and intervention. To this end, Magnetic Resonance Spectroscopy (MRS) is a non-invasive imaging technique that permits characterization of brain chemistry and is translatable from animal models to human AD subjects, permitting the monitoring of disease progression and treatment efficacy.The aim of this study was to assess longitudinal changes in neurochemistry and cognition in the TgF344-AD (Tg) rat model of AD under treatment conditions. MRS scans and behavioural testing were performed at 4, 10 and 16 months, providing insight into disease- and treatment-related change. Treatment consisted of early long (1-10months), early short (4-10months), or late (10-16months) administration of Naproxen, a common non-steroidal anti-inflammatory drug (NSAID), which has been shown to have beneficial effects on disease progression, but only when administered during pre-symptomatic stages of the disease(1–3). Preliminary results suggest altered metabolite levels exist in the TgF344-AD model despite absence of significant cognitive impairment, and the neurochemical response to treatment depends on treatment timing.Methods
Study Design: TgF344-AD rats (Tg) and wildtype (WT) littermates were split into 4 groups per genotype: no treatment (NT), early long treatment (ETlong), early short treatment (ET), or late treatment (LT). Groups contained between 16 and 24 rats (even number of males and females), for a total of 176 rats tested over three timepoints.Drug Treatment: Naproxen (Millipore Sigma, 615 ppm) was formulated into animal chow (Envigo) and groups received either normal chow or Naproxen chow ad libitum. Naproxen chow was administered from one-week post-weaning (~1 month) until 10 months of age, from 4 to 10 months, or from 10 to 16 months, for ETlong, ET, and LT groups, respectively.
Proton MRS acquisition and analyses: All 1H-MRS data was acquired on a 7 Tesla Bruker Biospec 70/30 scanner. A high-resolution anatomical image guided placement of a region of interest for localized MRS in the dorsal hippocampus. Automated localized shimming was performed using the FASTMAP method(4) prior to PRESS MRS acquisition (TR/TE = 3000/11ms). The FID-A toolkit was used to perform pre-processing and to generate a basis set for LCModel(5) analysis of MRS data. This basis set included nine macromolecule resonances simulated based on parameters obtained from metabolite-nulled spectra described previously(6). Concentrations are reported referenced to water. Concentrations were fit using a linear mixed effects model (Rv3.63; lmerTest_3.1-3; effects_4.2-0; ggplot2_3.3.2; lme4_1.1-25) with an interaction between age (modelled via second-order natural spline), genotype, and treatment, a fixed effect of sex, a random intercept per subject, and a weighting factor of the inverse absolute Cramer Rao Lower Bound for each metabolite.
Barnes Maze: Behavioural data were acquired in all groups except for the early short treatment paradigm, due to limitations in testing capacity. A shortened Barnes maze protocol was used to assess spatial learning and memory(7). Percent (%) time in target quadrant, % success, and path length were recorded during the probe trial and used to assess cognition. One-sample t-tests were used to determine if rats spent more than a chance amount of time (25%) in the target quadrant. % success was assessed using Kruskal-Wallis with Dunn post-hoc tests for group differences. Path length data were fit using linear regression with a fixed effect of group.
Results and Discussion
A representative MRS spectrum from a TgF344-AD rat at 4 months of age demonstrates the excellent spectral quality consistently obtained in this study (Figure 1B). Average water linewidth across all 513 scans was 9.07+/-0.74 Hz. Longitudinal MRS measurements in untreated TgF344-AD rats demonstrate this AD model recapitulates most neurochemical features of human AD(8–11), including increased total choline and decreased N-acetylaspartate/myo-inositol and Aspartate/Glutamate (Table 1,Figure 2). Tg rats also displayed decreased taurine and increased lactate. Despite neurochemical changes, significant cognitive deficits were not present in this model. 16-month Barnes Maze data (Figure 4), revealed Tg rats successfully spent greater than chance amount of time (25%) in the target quadrant, and displayed a comparable % success rate to WT rats. Analysis of path length revealed Tg rats explored the maze significantly less than WT rats, suggesting increased anxiety, a known component of AD in humans and rodent models(12,13). Naproxen treatment administered early but not late appears to mitigate disease-dependent changes in taurine and total choline (Figure 3). Unexpectedly, myo-inositol levels were not affected. Behavioural treatment effects assessed at 16-months were minor (Figure 4); ETlong and LT increased % success rate and path length in Tg rats, though this was only significant for path length, supporting the possible anti-anxiolytic effects of NSAIDs14. Overall, treatment effects on neurochemistry appear to depend on timing of administration, as shown in other NSAID studies(2,15,16), while behavioural analyses revealed a possible anxiety phenotype rather than cognitive impairment in the TgF344-AD model, which improves upon administration of Naproxen.Conclusion
These preliminary results confirm the value of MRS as a tool for monitoring disease progression and treatment response in a rat model of AD. Mitigation of disease-dependent neurochemical changes depends on timing of Naproxen administration, whereas behavioural analyses revealed possible anti-anxiolytic effects of both early and late Naproxen treatment.Acknowledgements
No acknowledgement found.References
1. Marjańska, M. et al. Treatment effects in a transgenic mouse model of Alzheimer’s disease: A magnetic resonance spectroscopy study after passive immunization. Neuroscience 259, 94–100 (2014).2. Choi, J.-K. et al. The effects of aging, housing and ibuprofen treatment on brain neurochemistry in a triple transgene Alzheimer’s disease mouse model using magnetic resonance spectroscopy and imaging. Brain Res. 1590, 85–96 (2014).3. Jantzen, P. T. et al. Microglial activation and beta -amyloid deposit reduction caused by a nitric oxide-releasing nonsteroidal anti-inflammatory drug in amyloid precursor protein plus presenilin-1 transgenic mice. J. Neurosci. 22, 2246–2254 (2002).4. Gruetter, R. Automatic, Localized in Vivo Adjustment of All First- and Second-Order Shim Coils. Journal of Magnetic Resonance in Medicine 29, 804–811 (1993).5. Provencher, S. W. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 14, 260–264 (2001).6. Fowler, C. F., Madularu, D., Dehghani, M., Devenyi, G. A. & Near, J. Longitudinal Quantification of Metabolites and Macromolecules Reveals Age- and Sex-Related Changes in the Healthy Fischer 344 Rat Brain. Cold Spring Harbor Laboratory 2020.04.29.069542 (2020) doi:10.1101/2020.04.29.069542.7. Attar, A. et al. A shortened Barnes maze protocol reveals memory deficits at 4-months of age in the triple-transgenic mouse model of Alzheimer’s disease. PLoS One 8, e80355 (2013).8. Marjańska, M., McCarten, J. R., Hodges, J. S., Hemmy, L. S. & Terpstra, M. Distinctive Neurochemistry in Alzheimer’s Disease via 7 T In Vivo Magnetic Resonance Spectroscopy. J. Alzheimers. Dis. 68, 559–569 (2019).9. Marjanska, M. et al. Monitoring disease progression in transgenic mouse models of Alzheimer’s disease with proton magnetic resonance spectroscopy. Proc. Natl. Acad. Sci. U. S. A. 102, 11906–11910 (2005).10. Nilsen, L. H., Melø, T. M., Saether, O., Witter, M. P. & Sonnewald, U. Altered neurochemical profile in the McGill-R-Thy1-APP rat model of Alzheimer’s disease: a longitudinal in vivo 1 H MRS study. J. Neurochem. 123, 532–541 (2012).11. Dedeoglu, A., Choi, J.-K., Cormier, K., Kowall, N. W. & Jenkins, B. G. Magnetic resonance spectroscopic analysis of Alzheimer’s disease mouse brain that express mutant human APP shows altered neurochemical profile. Brain Res. 1012, 60–65 (2004).12. Ferretti, L., McCurry, S. M., Logsdon, R., Gibbons, L. & Teri, L. Anxiety and Alzheimer’s disease. J. Geriatr. Psychiatry Neurol. 14, 52–58 (2001).13. Samaey, C., Schreurs, A., Stroobants, S. & Balschun, D. Early Cognitive and Behavioral Deficits in Mouse Models for Tauopathy and Alzheimer’s Disease. Front. Aging Neurosci. 11, 335 (2019).14. Makunts, T., Cohen, I. V., Lee, K. C. & Abagyan, R. Population scale retrospective analysis reveals distinctive antidepressant and anxiolytic effects of diclofenac, ketoprofen and naproxen in patients with pain. PLoS One 13, e0195521 (2018).15. Varvel, N. H. et al. NSAIDs prevent, but do not reverse, neuronal cell cycle reentry in a mouse model of Alzheimer disease. J. Clin. Invest. 119, 3692–3702 (2009).16. Breitner, J. C. et al. Extended results of the Alzheimer’s disease anti-inflammatory prevention trial. Alzheimers. Dement. 7, 402–411 (2011).Figures
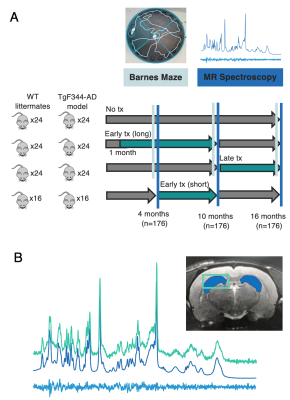
Figure 1: Visualization of study paradigm and spectroscopy data quality. A. MRS scans and Barnes Maze testing were performed in TgF344-AD and WT littermates at 4, 10, and 16 months. Rats were randomly separated into three groups treated with Naproxen: early long treatment, early short treatment, and late treatment. B. Localized 1H-MRS spectra from the hippocampus of a 4-month-old TgF344-AD rat acquired using the PRESS pulse sequence at 7T. Voxel placement is shown in the top right.
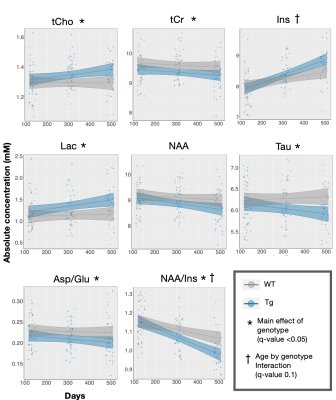
Figure 2. Longitudinal characterization of the neurochemical profile in the TgF344-AD rat reveals metabolic changes comparable to those seen in human Alzheimer’s disease. Presence of a genotype effect and/or age by genotype interaction was determined using linear mixed effects modelling. Each rat is depicted by an individual data point. The linear model used to fit the data is represented by a line of best fit and 95% prediction interval (shaded). Effects were significant if q value < 0.05.
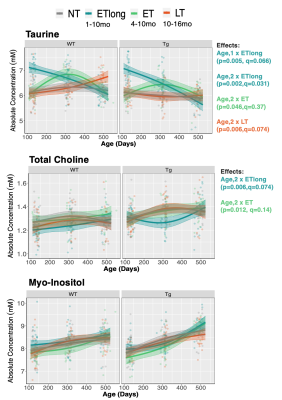
Figure 3: Early but not late treatment mitigates some disease-related metabolic changes. Three treatment paradigms were tested, with timing of Naproxen administration denoted in the figure legend. Linear mixed effects modelling was applied to examine age*genotype*treatment effects. Each rat is depicted by an individual data point. The linear model used to fit the data is represented by a line of best fit and 95% prediction interval (shaded). FDR correction applied at 5%.
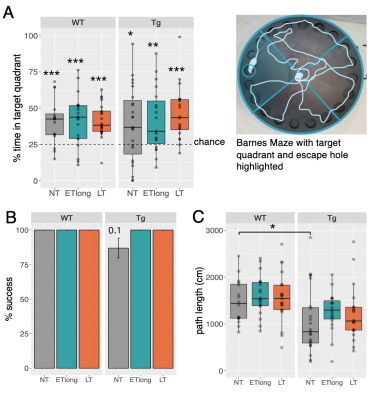
Figure 4: Barnes Maze analysis at 16-months reveals possible anxiety phenotype in Tg rats, which improves upon administration of Naproxen. A) One-sample t-tests determined if rats spent more than a chance amount of time (25%) in the target quadrant. Maze set-up with target quadrant highlighted. B) % success was assessed using Kruskal-Wallis with Dunn post-hoc tests for treatment group differences. C) Path length data were fit using linear regression with a fixed effect of genotype or group.
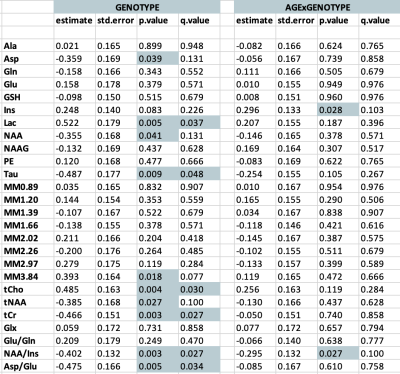
Table 1: Summary of linear mixed effects model results for 27 neurochemicals quantified longitudinally in untreated TgF344-AD rats. 1H-MRS spectra were acquired at 4, 10, and 16 months. Estimate represents the standardized beta or coefficient value for the variable of genotype or the interaction between age and genotype. Std.Error represents the standard error of the beta coefficient measurement. FDR correction applied at 5%. P and q values < 0.05 are denoted by shading.