1915
Hippocampal viscoelasticity is associated with risk of mild cognitive impairment1Department of Biomedical Engineering, University of Delaware, Newark, DE, United States, 2Dartmouth College, Hanover, NH, United States, 3Department of Communication Sciences & Disorders, University of Delaware, Newark, DE, United States, 4Swank Center for Memory Care and Geriatric Consultation, ChristianaCare, Wilmington, DE, United States, 5Kinesiology and Applied Physiology, University of Delaware, Newark, DE, United States
Synopsis
The health and integrity of the hippocampus is implicated in the development of mild cognitive impairment (MCI) and Alzheimer’s disease (AD). Neuroimaging techniques evaluate the characteristics of hippocampal health in vivo, including its size, diffusion properties, and blood flow. In this study, we show that the mechanical properties of the hippocampus, obtained through magnetic resonance elastography (MRE), are associated with an increased risk of MCI, when other techniques are not. Establishing risk based on practically relevant quantitative changes in neuroimaging variables can help identify participants likely to develop cognitive impairment and assist in establishing the effectiveness of treatment interventions.
Introduction
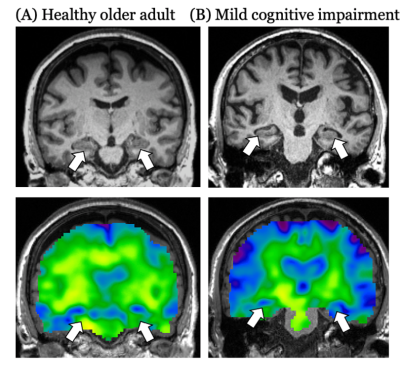
The neuropathological cascade that eventually leads to Alzheimer’s disease (AD) begins decades before the occurrence of clinical symptoms1. Novel biomarkers are urgently being developed to characterize the very early stages of the disease which will contribute to identifying causal mechanisms and the eventual development of effective disease-modifying therapies. Mild cognitive impairment (MCI) is a clinical syndrome that is typically a precursor to AD and thus the study of people with MCI can assist in understanding the progressive development of AD pathology. The hippocampus is one of the most studied brain regions due to its critical role in memory, and multiple neuroimaging techniques have been applied to characterize hippocampal integrity in cognitively healthy participants, and in MCI and AD. In this study, we examine hippocampal viscoelasticity outcomes from magnetic resonance elastography (MRE)2,3, which have emerged as a sensitive measure of microstructural tissue health, to determine sensitivity to MCI and utility in detecting this condition. We adopt a quantitative multimodal neuroimaging approach consisting of measures of hippocampal viscoelasticity (shear stiffness and damping ratio), as well as volume, perfusion, and diffusion – including neurite orientation and dispersion density imaging (NODDI) – for measurement of hippocampal health and integrity. The purpose of this study was to determine whether hippocampal MRI measures were predictive of an MCI diagnosis. Additionally, we sought to characterize the relative risk of MCI as a measure of effect size and establish risk profiles for a practically relevant unit change of each measure.Methods
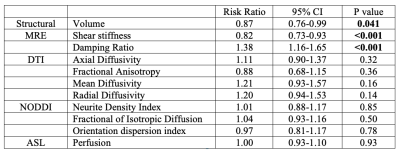
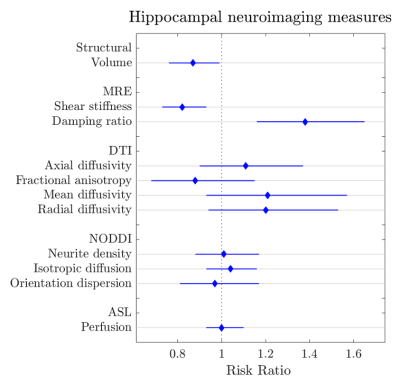
Twenty-three cognitively healthy older adults (OA) (mean age=69+6 years) and 7 participants with amnestic MCI (mean age=72+7 years) age were recruited; MCI was confirmed via scores on standardized tests and informant interview. Each participant underwent structural MRI, MRE, diffusion-weighted imaging, and pseudo-continuous arterial spin labeling. Hippocampal volumes were obtained from FreeSurfer4 and the same hippocampal binary masks from each participant were coregistered to the other imaging outputs using ANTS5 to obtain quantitative measures from each modality. Eleven different measures of hippocampal structure and integrity were obtained for each participant (Table 1). As the outcome of MCI was frequent (23%), a modified Poisson regression (with a robust error variance) approach was used to estimate the relative risk (RR)6 of MCI compared to OA based on each of the 11 neuroimaging measures. Each model was adjusted for age and sex. Statistical analyses were performed in STATA 16.1.Results
The relative risk ratios and 95% CI for MCI based on hippocampal neuroimaging measures are provided in Table 1 and shown in Figure 1. Only hippocampal volume, MRE shear stiffness, and MRE damping ratio were predictive of MCI. Results indicated that for every 0.1 cm3 decrease in hippocampal volume, the likelihood of having MCI increases by 13%. For MRE, a 0.1 kPa decrease in hippocampal stiffness and 0.01 increase in the damping ratio increases the likelihood of MCI by 18% and 38%, respectively. None of the other imaging metrics presented a significant risk factor in the presentation of MCI.Discussion and conclusions
Hippocampal size, stiffness, and damping ratio were predictive of mild cognitive impairment. A smaller volume, lower stiffness, and greater damping ratio each represented an increased risk of possessing MCI. While loss of hippocampal volume is traditionally used to assess the extent of neurodegeneration associated with AD and used to inform inclusion into disease-modifying clinical trials, our results demonstrate that small changes in MRE measures are associated with the greatest risk of MCI. As a result, MRE may be a sensitive biomarker for detecting changes to tissue that have cognitive consequences7,8 and could be used to monitor the impact of targeted treatment interventions9. Healthy individuals deemed to be most at risk should be followed up over time to assess whether the aforementioned hippocampal measures (i.e., a decrease in hippocampal stiffness) predict subsequent MCI or AD. Future work with a larger sample will seek to confirm a minimal set of parameters most associated with MCI.Acknowledgements
This work was supported in part by grants NIH/NIA R01-AG058853, NIH/NIBIB R01-EB027577 and K01-AG054731.References
1. Reiman, E. M., Quiroz, Y. T., Fleisher, A. S., Chen, K., Velez-Pardo, C., Jimenez-Del-Rio, M., et al. Brain imaging and fluid biomarker analysis in young adults at genetic risk for autosomal dominant Alzheimer’s disease in the presenilin 1 E280A kindred: a case-control study. Lancet Neurol. 11, 1048–1056 (2012).
2. Muthupillai, R. & Ehman, R. L. Magnetic resonance elastography. Science. 269, 1854–1857 (1995).
3. Hiscox, L. V, Johnson, C. L., Barnhill, E., McGarry, M. D. J., III Huston, J., van Beek, E. J. R., et al. Magnetic resonance elastography (MRE) of the human brain: technique, findings and clinical applications. Phys Med Biol 61, 401–437 (2016).
4. Fischl, B., Salat, D. H., Busa, E., Albert, M., Dieterich, M., Haselgrove, C., et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron, (2002).
5. Avants, B. B., Tustison, N. J., Song, G., Cook, P. A., Klein, A. & Gee, J. C. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage 54, 2033–2044 (2011).
6. Ranganathan, P., Aggarwal, R. & Pramesh, C. S. Common pitfalls in statistical analysis: Odds versus risk. Perspect. Clin. Res. 6, 222–224 (2015).
7. Schwarb, H., Johnson, C. L., McGarry, M. D. J. & Cohen, N. J. Medial temporal lobe viscoelasticity and relational memory performance. Neuroimage 132, 534–541 (2016).
8. Johnson, C. L., Schwarb, H., Horecka, K. M., McGarry, M. D. J., Hillman, C. H., Kramer, A. F., et al. Double dissociation of structure-function relationships in memory and fluid intelligence observed with magnetic resonance elastography. Neuroimage 171, 99–106 (2018).
9. Sandroff, B. M., Johnson, C. L. & Motl, R. W. Exercise training effects on memory and hippocampal viscoelasticity in multiple sclerosis: a novel application of magnetic resonance elastography. Neuroradiology 59, 61–67 (2017).
Figures