1913
Relating Non-invasive Imaging Features of Vascular Aging in the Rodent Brain and Aorta1Radiology, University of New Mexico, Albuquerque, NM, United States
Synopsis
Diseases with age as the primary risk factor increase in prevalence as life expectancy increases. Aging is implicated in cardiovascular (CV) disease and neurodegeneration. As the stiffness of the proximal aorta increases, damaging pulses of pressure have been postulated to propagate into the brain. The purpose of this study was to relate vascular and brain pathology through non-invasive measures in normal and hypertensive rodents. Brain pathology was detected by multiple MRI contrast mechanisms, while specific vascular parameters were derived from ultrasound in the aorta. Non-invasive measures relating brain and vascular aging could aid in the future development of therapeutic strategies.
INTRODUCTION:
Environmental and lifestyle choices have an impact on brain aging and in particular, cardiovascular (CV) health impacts the quality and amount of blood flowing into the brain. Vascular stiffness in particular is believed to be a driving force for age-related pathology. In the brain, hypertension and vascular stiffness are related to the incidence of white matter (WM) hyperintensities, cerebral microbleeds (CMBs), and microstructural damage (1–3). The purpose of this study was to determine the timing and relationship of specific vascular parameters derived from non-invasive ultrasound to brain aging determinants in MRI with multiple contrast mechanisms.METHODS:
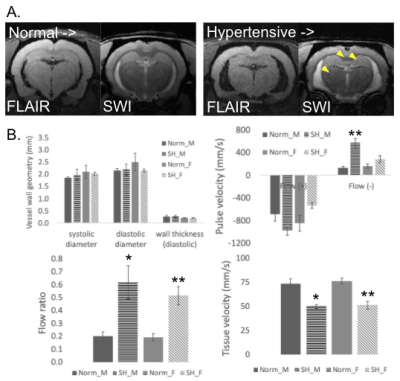
Age-matched normal (WKY) and hypertensive rats (SHR) were purchased from Envigo (Indianapolis, IN) at 14 months of age. This study was performed using equal numbers of male and female animals and the study was approved by IACUC. Baseline MRI (7T BioSpec; Bruker, Billerica, MA) was performed on 24 animals from 14 to 19 months of age. This MRI method included Fluid Attenuated Inversion Recovery (FLAIR) for white matter lesions, T2W MRI for brain structure and fluid presence, Susceptibility Weighted Imaging (SWI) for CMBs detection, and generalized Q-space imaging (GQI) for the detection of microstructural diffusion changes in the brain. Baseline MRI was followed by ultrasound (Vevo LAZR-X; Fujifilm, Japan) of the aortic arch for structural (M-mode), doppler pulse velocity (PV), and doppler tissue velocity (TV).RESULTS:
When comparing structural MRI, SHR had larger ventricle volumes, indicating a reduction in brain volume. MRI was used to identify lesions including SWI for hypointensities (CMBs) and T2/FLAIR to identify hyperintensities/ hypointensities, respectively (acute lesions). In SHR, 11/12 animals presented with one or both lesion types, while in WKY 7/12 animals presented with one or both lesions. The thalamus was the most common location for lesion presentation, followed by the hippocampus (Figure 1.A). GQI identified white matter diffusion abnormalities when comparing SHR and WKY using quantitative measures, particularly with anisotropy metrics in the corpus callosum (CC). Apparent diffusion (AD) was also significantly decreased in the female SHR CC compared to female WKY. Using ultrasound, changes in flow parameters in the SHR were observed (Figure 1.B) in terms of increased reverse flow with PV and the calculated flow ratio (reverse/ forward flow) in SHR. Moreover, TV was substantially reduced in SHR compared to WKY of both sexes. Ultrasound of the aortic arch did not identify any structural differences in terms of systolic or diastolic aorta vessel lumen diameter or vessel wall thickness.DISCUSSION:
MRI and ultrasound were used to non-invasively determine changes related to brain and systemic vascular aging, respectively. SHR develop hypertension at an early age that persists throughout their lifetime, compared to WKY that will experience increased vascular stiffness and so increased blood pressure with aging. While many animals from both SHR and WKY breeds presented with brain pathology, brain aging was accelerated in the SHR as shown by increased ventricle volumes, more microbleeds or acute lesions in the thalamus or hippocampus, and microstructural diffusion changes in the CC. The remaining question being addressed by this study was ‘what particular vascular parameters beyond hypertension may play a role in the acceleration of brain aging?’Using ultrasound, it was possible to non-invasively quantify vascular parameters in the aortic arch. The first important observation was that lumen diameter or vessel wall thickness was not substantially changed in age and sex matched SHR vs. WKY. With many dietary CV diseases associated with vascular aging, such as atherosclerosis, changes in vessel wall thickness are expected. With hypertensive aging in SHR, changes in smooth muscle cell (SMC) properties along with collagen and elastin extracellular matrix (ECM) properties are instead more likely at play (4). In doppler PV, it was found that forward flow in the aortic arch was relatively consistent, while reverse flow was increased in SHR, either in terms of absolute velocity in male SHR or the ratio of forward to reverse velocity in both sexes. Previous studies with ultrasound have found that aortic stiffness determines diastolic blood flow reversal in the aorta (5). Second, doppler TV was reduced in SHR of both sexes, implying an inability of the vessel wall to contract rapidly in response to pressure being applied from the heart. Once again, this could be due to SMC or ECM changes with aging and hypertension that do not allow the tissue to respond normally. Previous studies have determined that vascular stiffness increased with aging in rodents (6) and it is likely that SHR also had increased vascular stiffness. In composite, increased reverse flow ratio and lower TV in SHR could result in reduced tissue oxygenation or higher PV propagation, respectively, that accelerated the damage to the brain.
CONCLUSION:
Hypertension accelerated brain aging in SHR when compared to normal WKY rats in terms of structural changes, CMBs or other lesion presence, and altered tissue diffusion properties. Ultrasound was used to determine possible vascular mechanisms of accelerated brain aging with hypertension. Ultrasound findings indicated that changes in forward and reverse blood flow pulsation and reduced vascular tissue responses are potential mechanisms of accelerated brain aging with hypertension. The ability to relate brain and vascular aging non-invasively could lead to future therapeutic strategies.Acknowledgements
The author would like to thank Yirong Yang, PhD for help with acquisition of the MRI data, the Brain and Behavior Health Institute (BBHI) pilot program for funding, and the Cardiovascular and Metabolic Disease (CVMD) program for funding.References
1. Iadecola C. The pathobiology of vascular dementia. Neuron 2013;80:844–866.
2. de Roos A, van der Grond J, Mitchell G, Westenberg J. Magnetic Resonance Imaging of Cardiovascular Function and the Brain: Is Dementia a Cardiovascular-Driven Disease? Circulation 2017;135:2178–2195.
3. Akoudad S, Wolters FJ, Viswanathan A, et al. Association of Cerebral Microbleeds With Cognitive Decline and Dementia. JAMA Neurol. 2016;73:934–943.
4. Sehgel NL, Sun Z, Hong Z, et al. Augmented vascular smooth muscle cell stiffness and adhesion when hypertension is superimposed on aging. Hypertension 2015;65:370–377.
5. Hashimoto J, Ito S. Aortic stiffness determines diastolic blood flow reversal in the descending thoracic aorta: potential implication for retrograde embolic stroke in hypertension. Hypertension 2013;62:542–549.
6. Nicholson CJ, Singh K, Saphirstein RJ, et al. Reversal of Aging-Induced Increases in Aortic Stiffness by Targeting Cytoskeletal Protein-Protein Interfaces. J. Am. Heart Assoc. 2018;7:e008926.
Figures