1898
Diffusion-Weighted Imaging of the Abdomen using Echo Planar Imaging with Compressed SENSE (EPICS)1Department of Radiology, Gifu University, Gifu, Japan, 2Department of Radiology Services, Gifu University Hospital, Gifu, Japan, 3Philips Japan, Tokyo, Japan, 4Department of Frontier Science for Imaging, Gifu University, Gifu, Japan
Synopsis
Diffusion-weighted imaging (DWI) is one of the important sequences in abdominal magnetic resonance (MR) imaging, especially in unenhanced examination. Recently, echo planar imaging with Compressed SENSE (EPICS) has been introduced and it can reduce noise-like artifacts and improve image quality. In this study, we compared qualitative and quantitative imaging parameters between conventional echo planar DWI (C-EPI-DWI) and EPICS. Our results showed that EPICS significantly reduce noise-like artifacts and improve the accuracy of ADC values compared with C-EPI-DWI.
Purpose
Conventional diffusion-weighted imaging with echo planar imaging (C-EPI-DWI) has limited spatial resolution because of its high sensitivity to B0 inhomogeneities. Additionally, it often suffers from increased noise-like artifacts on the center of the images due to the high geometry factor when large acceleration factors are used1, 2. Recently, a combination of parallel imaging and compressed sensing (Compressed SENSE) has been developed to reduce the acquisition time and image noise. In the present study, we attempt to evaluate the feasibility of echo planar imaging with Compressed SENSE (EPICS) of the abdomen3 and compare it with C-EPI-DWI.Materials and Methods
This prospective study was approved by our Institutional Review Board, and written informed consent was obtained from all patients. Nineteen patients (8 men and 11 women, mean age, 65.2 ± 14.1 years; age range, 34–88 years) with suspected upper abdominal diseases underwent MR imaging between October 2020 and November 2020 were included. Using a 3T MR system (Ingenia 3.0T CX; Philips Medical Systems, Best, the Netherlands) equipped with a 32-channel digital coil, we performed MR imaging of the abdomen. DWI was obtained using free-breathing two-dimensional fat-suppressed single-shot echo-planar sequence and EPICS (repetition time/echo time, 5,000/64 msec; matrix, 192 × 154; field of view, 38 × 30 cm; SENSE or CS factor, 3.0; NSA = 2.0; b factors, 0, 200, and 800 s/mm2; section thickness, 7 mm with 0-mm intersection gap; acquisition time for 30 sections, 1 min 45 s). Two radiologists reviewed apparent diffusion coefficient (ADC) maps and assigned confidence score for image noise, liver contour, and pancreas contour using a 5-poing scale: 5 = excellent, 4 = good; 3 = acceptable, 2 = suboptimal, and 1 = unacceptable. The radiologists also measured mean ADC value and standard deviation (SD) using region-of-interests placing on the liver, pancreas, and spleen. We calculated the Coefficient of Variation (CV) in ADC of the liver, pancreas, and spleen using the following equation: CV = SD / average ADC. The Wilcoxon test was conducted to compare the qualitative and quantitative parameters between C-EPI-DWI and EPICS. Inter-observer variability in qualitative and quantitative measurements was assessed using the ĸ statistic and the intraclass correlation coefficient (ICC), which measures the degree of agreement between two radiologists. A ĸ value and ICC of ≤ 0.20 was interpreted as slight agreement, 0.21–0.40 as fair agreement, 0.41–0.60 as moderate agreement, 0.61–0.80 as substantial agreement, and ≥ 0.81 as almost perfect agreement. A P value of less than 0.05 was considered to be significant.Results
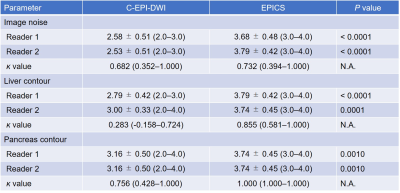
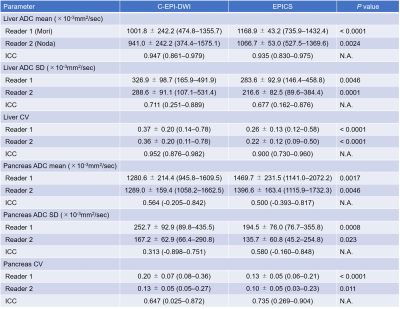
Mean confidence scores for image noise, liver contour, and pancreas contour were significantly higher in EPICS than in C-EPI-DWI in both radiologists (P ≤ 0.001). The ĸ values ranged from 0.28 to 1.00, indicating fair to almost perfect agreement between the two radiologists (Table 1). The mean ADC values of the liver and pancreas were significantly higher in EPICS than in C-EPI-DWI in both radiologists (P < 0.001–0.0046). On the other hand, the mean SD and CV of the liver and pancreas were significantly lower in EPICS than in C-EPI-DWI in both radiologists (P < 0.001–0.023). No significant difference was found in the ADC value, SD, and CV of the spleen between C-EPI-DWI and EPICS (P = 0.14–1.00). The ICCs ranged from 0.31 to 0.95, indicating fair to almost perfect agreement between the two radiologists (Table 2).Discussion
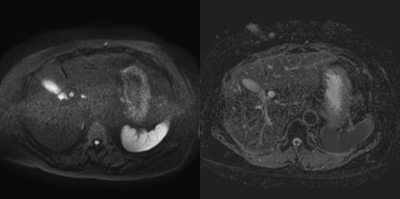
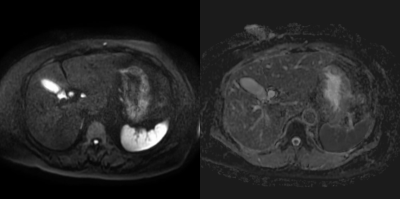
Our study demonstrated that EPICS could reduce image noise qualitatively and quantitatively. The mean ADC values of the liver and pancreas were significantly higher, and the CV were significantly lower in EPICS compared with C-EPI-DWI. It indicated that EPICS could provide more accurate ADC values with high reproducibility and robustness. The mean ADC values in EPICS were closer to the values reported in the previous literature4 compared with those in C-EPI-DWI. This previous literature reported that the ADC value of normal pancreas was 1488 ± 185 ×10-3mm2/sec. In C-EPI-DWI, noise-like artifacts on the center of the images were sometimes seen (Figure 1). In contrast, these artifacts were not seen in EPICS even though we did not have enough cases in this preliminary study (Figure 2). We believe that our result was highly affected by the presence or absence of severe noise over the images and EPICS could reduce image noise. In conclusion, EPICS was feasible in abdominal MR imaging and significantly improved image quality compared with C-EPI-DWI.Acknowledgements
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.References
1. Patricia N, et al. Parallel Imaging Artifacts in Body Magnetic Resonance Imaging. Can Assoc Radiol J. 2009;60: 91–98.
2. Yanasak NE, et al. MR imaging artifacts andparallel imaging techniques with calibration scanning: a new twist on old problems. Radiographics. 2014;34:532-48.
3. Yoneyama M, et al. Noise Reduction in Prostate Single-Shot DW-EPI utilizing Compressed SENSE Framework. Proc Intl Soc Mag Reason Med. (2019) 1634.
4. Kamisama T, et al. Differentiation of Autoimmune Pancreatitis From Pancreatic Cancer by Diffusion-Weighed MRI. Am J Gastroenterol. 2010 Aug;105(8):1870-5.
Figures