1889
Modeling Central Olfactory Network Alteration in Type 2 Diabetes Mellitus: From Primary to Advanced Cortex1Drum Tower Hospital, The Affiliated Hospital of Nanjing University Medical School, Nanjing, China, 2Philips Healthcare, Shanghai, China
Synopsis
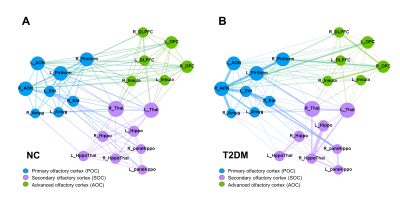
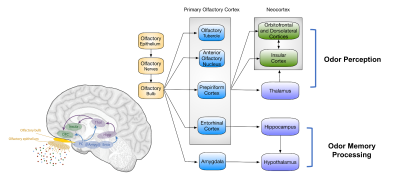
Type 2 diabetes mellitus (T2DM) is associated with olfactory dysfunction and cognitive decline. We proposed one functional connectivity (FC) model that links the primary olfactory cortex (POC) to advanced cognition related cognitive brain area. The whole olfactory network was divided into three sub-networks: POC consists of the bilateral anterior olfactory nucleus, piriform cortex, entorhinal cortex, and amygdala; secondary olfactory cortex (SOC) include the bilateral hippocampus, parahippocampus, thalamus, and hypothalamus; advanced olfactory cortex (AOC) consists of bilateral orbitofrontal cortex, insula and dorsolateral prefrontal cortex(DLPFC). Our results suggested that FC was heterogeneously disrupted in the POC-SOC-AOC olfactory pathway in T2DM.
Introduction
Type 2 diabetes mellitus (T2DM) is reported to be associated with olfactory dysfunction and cognitive decline. However, how olfactory neural circuit abnormalities involve cognitive impairment in diabetes remains unclear. To investigate olfactory network alterations and the mediation of odor perception and cognition in patients with T2DM, we proposed one functional connectivity (FC) model that links the primary olfactory cortex (POC) to advanced cognition related cognitive brain area.Methods
108 right-handed T2DM patients (age: 52.3±9.0, 59 male and 49 female) and 58 normal control (age: 54.2±9.6, 26 male and 32 female) were included. Resting-state functional MRI (rs-fMRI) data, olfactory behavior test score, and cognitive assessment scales were acquired from each participant. In this study, we applied the region of interest (ROI)-wise FC analyses to investigate the relationship between POC and neocortical cognition-related areas. We divided the whole olfactory network into three sub-networks: POC consists of the bilateral anterior olfactory nucleus, piriform cortex, entorhinal cortex, and amygdala; secondary olfactory cortex (SOC) include the bilateral hippocampus, parahippocampus, thalamus, and hypothalamus; advanced olfactory cortex (AOC) consists of the bilateral orbitofrontal cortex, insula, and dorsolateral prefrontal cortex(DLPFC). The network analysis was introduced to generate the network pattern and do group comparison.Results
There were no significant differences in age (p=0.218), sex (p=0.184), and education (p=0.188) between the two groups. Compared with the normal control, patients with T2DM demonstrated significantly lower olfactory threshold score (p=0.002) and decreased cognitive function, especially in recalled memory (p=0.005) and executive function (p=0.015). In the normal group, the inner and interconnections in the three sub-networks were well organized. However, the network integrality was disrupted in T2DM patients. There were significant decreased FC within and between these three sub-networks. Notably, the most severe FC decline was within the POC sub-network, followed by decreased FC between the POC and the SOC, the minimal disruption connections were between POC and AOC sub-network.Conclusion
The current study found FC was heterogeneously disrupted in the POC-SOC-AOC olfactory pathway in T2DM, revealed distinct FC decline between subregions in POC and advanced cognitive brain networks. Our results suggested that there could be some compensation mechanisms underlying the progressive disconnection from the primary cortex to the advanced cortex within the olfactory network. These may help explain cognitive dysfunction and its progression in diabetes.Acknowledgements
NoneReferences
- Mandairon, N., F. Kermen, C. Charpentier, J. Sacquet, C. Linster, and A. Didier. 2014. 'Context-driven activation of odor representations in the absence of olfactory stimuli in the olfactory bulb and piriform cortex', Front Behav Neurosci, 8: 138.
- Saive, Anne-Lise, Jean-Pierre Royet, and Jane Plailly. 2014. 'A review on the neural bases of episodic odor memory: from laboratory-based to autobiographical approaches', Frontiers in behavioral neuroscience, 8.
- Sanke, H., T. Mita, H. Yoshii, A. Yokota, K. Yamashiro, N. Ingaki, T. Onuma, Y. Someya, K. Komiya, Y. Tamura, T. Shimizu, C. Ohmura, A. Kanazawa, Y. Fujitani, and H. Watada. 2014. 'Relationship between olfactory dysfunction and cognitive impairment in elderly patients with type 2 diabetes mellitus', Diabetes Res Clin Pract, 106: 465-73.
- Zald, D. H., and J. V. Pardo. 2000. 'Functional neuroimaging of the olfactory system in humans', Int J Psychophysiol, 36: 165-81.