1887
Disrupted resting-state salience network in type 2 diabetes with and without mild cognitive impairment1Department of MRI, Shaanxi Provincial People’s Hospital, Xi'an, China, 2Xi'an Medical University, Xi’an, China, 3Philips Healthcare, Xi'an, China
Synopsis
To investigate the underlying mechanisms of cognitive decline in patients with diabetes, data-driven independent component analysis (ICA) was applied to resting-state fMRI images from 34 type 2 diabetes mellitus (T2DM) patients with normal cognition (DMCN) and 31 T2DM patients with cognitive impairment (DMCI), and 31 healthy controls (HC) to identify the difference in salience network (SN). The resting-state functional connectivity (rs-FC) alteration are different and complicated among HC, DMCN and DMCI groups, and also are correlated to neuropsychological scores, indicated that altered SN rs-FC in T2DM patients are closely related to cognitive impairment.
Introduction
The global prevalence of T2DM has been rapidly increasing[1]. Patients with T2DM showed significant decreases in information processing speed, attention, and executive function[2], but the exact mechanism remains unknown. Impaired SN rs-FC could accelerate cognitive impairment in Alzheimer's disease (AD) patients, which is mainly reflected in reduced ability to distinguish environmental stimuli and extensive attention dysfunction[3]. The neuropathological mechanisms of cognitive impairment are similar in T2DM and AD patients[4], but no specific studies investigated the changes in SN with T2DM. Data-driven ICA provides a good way for the individual analysis of different functional networks in the whole brain. In this study, the ICA was adopted to reveal the changes of SN and its influence on cognitive function under different cognitive states of T2DM.Methods
MRI data were obtained from 34 DMCN, 31 DMCI and 31HC on a 3.0-Tesla scanner (Ingenia, Philips healthcare, the Nertherlands) using a 16-channel phased-array head coil. Sagittal 3-dimensional T1-weighted images were acquired with the following parameters: TR = 7.5 ms, TE = 3.5 ms, FA = 8°, FOV = 250 mm × 250 mm, matrix = 256 × 256, slice thickness = 1 mm, no gap, and 328 sagittal slices. Resting-state functional BOLD images were obtained by using a gradient-echo planar sequence with the following parameters: TR = 2000 ms, TE = 30 ms, slices =34, thickness = 4 mm, gap =0 mm, FOV = 230 mm × 230 mm, matrix = 128 × 128, FA = 90°, and 200 volumes. We also collected the clinical and neuropsychological test information. Data preprocessing were performed by DPABI 3.0 and GIFT based on Matlab software (Mathworks, Natick, Massachusetts). One-way ANOVA was utilized to compare the clinical features, neuropsychological scores and the SN rs-FC across the three groups. GRF correction and least significant difference (LSD) were used to perform post hoc comparisons.Results
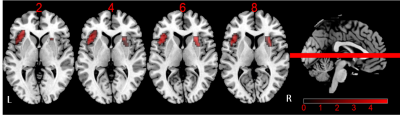
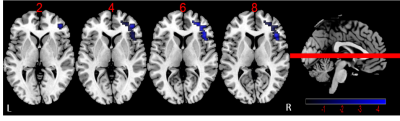
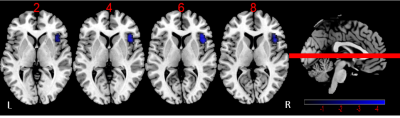
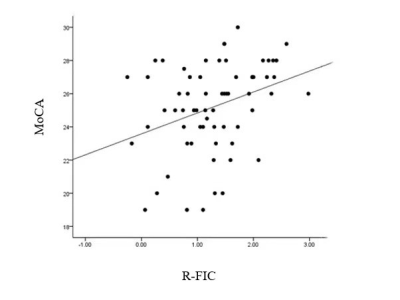
There are no significant difference for age, gender and education level among HC, DMCN and DMCI groups (P > 0.05). Compared to the HC group, the T2DM group had increased fasting blood glucose, glycated hemoglobin, Beck Depression Inventory and Trail-Making Test part A scores (all Ps < 0.05). We found rs-FC in the right fronto-insular cortex (FIC) and putamen of the SN with significant difference by ANOVA. Between-group analysis demonstrated DMCN group displayed increased rs-FC in the left FIC, as well as right dorsal anterior insula and putamen compared with HC (Figure 1); DMCI group showed decreased rs-FC in the right FIC compared with HC (Figure 2). Compared with DMCN group, DMCI group showed decreased rs-FC in the right FIC (Figure 3). In addition, the rs-FC of the right FIC were significantly correlated with Montreal Cognitive Assessment (MoCA) scores for all the T2DM subjects (r = 0.334, P = 0.007, Figure 4).Discussion
This study found that the abnormality of connectivity of SN may precede the abnormality of clinical cognitive dysfunction in T2DM patients, suggesting that patients may maintain relatively normal cognition through the compensatory mechanism of increased FC of the dorsal anterior insula of SN. The neuropathological mechanisms of T2DM and AD cognitive impairment are similar and both manifested as the amyloid-beta and tau deposition[5]. T2DM and MCI are both predisposition for AD, in addition, insulin resistance and hyperglycemia in diabetic patients also accelerate the amyloid-beta deposition in SN core nodes. Studies have found that the accelerated amyloid-beta deposition could lead to premature interruption of the recruitment of compensatory frontal processes[6], which may be the reason of DMCI patients showed reduced FC of the right FIC in SN relative to both HC and DMCN. FIC plays an important role in maintaining normal cognition includes the recognition and detection of internal and external salient stimuli, the completion of attention capture[7], and the adjustment of the relationship between CEN and DMN[8]. He et al. found that the bilateral FIC decreased intra-SN and associated with cognitive dysfunction in AD group[3]. Our study found that the right FIC functional connection in T2DM patients was positively correlated with MoCA scores. Therefore, we speculate that the altered FC of the right FIC may reflect the degree of cognitive impairment in T2DM patients.Conclusion
To our knowledge, this is the first study to discuss different FC patterns in SN of T2DM, we found that the SN pattern in DMCN and DMCI was different, the SN may have a dynamic change process from compensatory to decomposable in T2DM patients, and right FIC may be a potential biological marker for the evaluation of cognitive dysfunction in T2DM.Acknowledgements
This research was supported by the National Natural Science Foundation of China (81270416), the Key Research and Development Program of Shaanxi Province of China (2018ZDXM-SF-038), and the Social Development Science and Technology Research Project of Shaanxi Province of China (2019SF-131).References
[1] Aziz Z, Absetz P, Oldroyd J, et al. A systematic review of real-world diabetes prevention programs: learnings from the last 15 years[J]. Implement Sci, 2015, 10: 172.
[2] Karvani M, Simos P, Stavrakaki S, et al. Neurocognitive impairment in type 2 diabetes mellitus[J]. Hormones (Athens), 2019, 18 (4): 523-534.
[3] He X, Qin W, Liu Y, et al. Abnormal salience network in normal aging and in amnestic mild cognitive impairment and Alzheimer's disease[J]. Hum Brain Mapp, 2014, 35 (7): 3446-3464.
[4] Willette AA, Bendlin BB, Starks EJ, et al. Association of Insulin Resistance With Cerebral Glucose Uptake in Late Middle-Aged Adults at Risk for Alzheimer Disease[J]. JAMA Neurol, 2015, 72 (9): 1013-1020.
[5] Infante-Garcia C, Ramos-Rodriguez JJ, Galindo-Gonzalez L, et al. Long-term central pathology and cognitive impairment are exacerbated in a mixed model of Alzheimer's disease and type 2 diabetes[J]. Psychoneuroendocrinology, 2016, 65: 15-25.
[6] Lin F, Ren P, Lo RY, et al. Insula and Inferior Frontal Gyrus' Activities Protect Memory Performance Against Alzheimer's Disease Pathology in Old Age[J]. J Alzheimers Dis, 2017, 55 (2): 669-678.
[7] Kurth F, Zilles K, Fox PT, et al. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis[J]. Brain Struct Funct, 2010, 214 (5-6): 519-534.
[8] Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks[J]. Proc Natl Acad Sci U S A, 2008, 105 (34): 12569-12574.
Figures