1879
Intenso MRE: 3D volumetric GRE-based MR Elastography of the liver in a single breath-hold1King's College London, London, United Kingdom, 2INSERM U1148, LVTS, University Paris Diderot, Paris, France, 3MR Research Collaborations, Siemens Healthcare Limited, Frimley, United Kingdom, 4MR Research Collaborations, Siemens Healthcare Limited, Melbourne, Australia, 5MR Application Predevelopment, Siemens Healthcare GmbH, Erlangen, Germany
Synopsis
The increasing use of MR elastography (MRE) for the quantification of liver fibrosis and inflammation demands a rapid and accurate 3D MRE sequence. 3D MRE usually requires multiple breath-holds, which prolongs acquisition times and introduces misalignment between the different acquisitions. We propose Intenso, a novel GRE-MRE sequence that enables 3D motion-encoding for volumetric MRE of the liver in a single breath-hold. The sequence combines simultaneous multi-slice excitation (SMS), Hadamard motion encoding and a multi-shot GRE-MRE sequence resulting in a total acquisition time of 17 seconds. In-vivo results are shown in three healthy volunteers and compared to a well-established GRE-MRE sequence.
Introduction
Magnetic Resonance Elastography (MRE) enables safe non-invasive estimation of biomechanical parameters in tissues. Biomechanical parameters (elasticity and viscosity) receive a growing clinical interest, especially in the domain of liver fibrosis quantification1,2. Liver fibrosis is triggered by prolonged inflammation; thus, the simultaneous staging of both inflammation and fibrosis is mandatory for efficient patient management. 3D MRE-GRE sequences have demonstrated that a combination of elasticity and viscosity allows to stage non-invasively both, i.e. the fibrosis grade and the inflammation grade1.However, current 3D volumetric liver MRE-GRE sequences require multiple breath-holds (BH), usually 4 BH. This hampers clinical acceptance and reduces the quality and hence precision of the viscoelastic parameters due to imperfect geometrical matching of the different breath-holds.
In this work, we propose a simultaneous multi-slice (SMS) rapid GRE-MRE sequence termed Intenso that enables the acquisition of a 3D volumetric MRE liver dataset within 17 seconds. We will compare results obtained using this single-BH approach with a multiple-BH sequence (eXpresso3) in volunteer experiments.
Methods
The design of the Intenso sequence combines an in-plane GRAPPA-based SMS acquisition4 and a previously developed multi-shot GRE-MRE sequence (Ristretto5) which incorporates delays within imaging shots in order to synchronize slice and vibrational wave phase acquisition with the mechanical vibration. This allows the interleaved acquisition of multiple slices, wave phases and motion encodings in a single concatenated measurement.The SMS acquisition was achieved by employing dual-band RF pulses with different phase cycles (slice 1: 0, 0, 0, 0…, slice 2: 0, π, 0, π…) in the phase-encoding direction that excite two slices simultaneously resulting in an image where one slice is shifted in the phase oversampling region of the other slice6. Additionally, a Hadamard motion encoding scheme7 is used to greatly increase motion sensitivity and thereby counteracting the g-factor SNR penalty due to the SMS acquisition.
The proposed Intenso sequence was implemented on a 3T system (Biograph mMR, Siemens Healthcare, Erlangen, Germany). Liver experiments were performed with 60Hz actuation frequency8 in three healthy volunteers with four wave-phase offsets and four Hadamard motion encoding combinations (20 mT/m). Imaging parameters were 8 slices, 4mm isotropic resolution, an 96 x 66 acquisition matrix acquired with a total acceleration factor of 5 (including in-plane and SMS acceleration) resulting in an FOV of 386 x 264 x 32 mm3, TR=9.38ms, TE=7.38ms. This resulted in a BH duration of 17 seconds.
Hadamard decoding was performed for each coil channel prior to coil combination. MRE reconstruction was performed using the curl operator to remove the compressional wave followed by a direct inversion of the complex wave equation9.
Intenso was compared with an eXpresso sequence with matching imaging parameters but acquired in 4 BHs of 21 seconds each.
Results and Discussion
Figure 1 shows the plots of the total amplitude of the complex displacement field measured with eXpresso and Intenso in the three volunteers. A simple linear regression analysis was carried out to quantify the regression slopes. The plots show a good agreement between the two sequences, with regression slopes ranging between 0.8966 and 1.0003.Figure 2 demonstrates a comparison for the shear velocity in the liver between Intenso and eXpresso in volunteer 1. The shear velocity is matching closely, 1.35 ± 0.15 m/s for eXpresso and 1.28 ± 0.11 m/s for Intenso. Volunteers 2 and 3 showed a similar agreement. (Volunteer2: 1.31 ± 0.13 m/s (eXpresso) and 1.25 ± 0.13 m/s (Intenso). Volunteer3: 1.37 ± 0.18 m/s (eXpresso) and 1.41 ± 0.21 m/s (Intenso)).
A repeatability test was carried out for the volunteers 2 and 3. Both volunteers were scanned twice using eXpresso and Intenso. The plots of the total amplitude of the complex displacement field measured in the two tests are shown in figure 3. The tests were repeatable, with regression slopes ranging between 0.8916 and 1.0073.
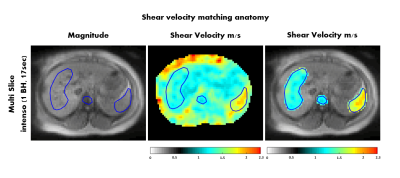
In figure 4, the shear velocity measured in volunteer 1 using Intenso is matched with the abdominal anatomy. As expected, the shear velocity is higher in the spleen compared to the liver (1.67 ± 0.19 m/s (spleen) and 1.28 ± 0.11m/s (liver)).
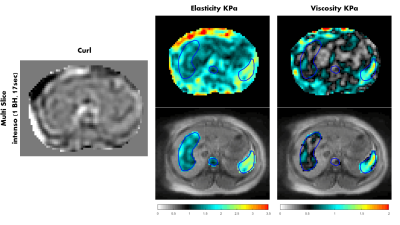
The elasticity and viscosity maps are shown for volunteer 1 in figure 5 along with one component of the curl of the displacement field. The spleen has higher elasticity and viscosity values than the liver (Elasticity: 2.18 ± 0.48 KPa (spleen) and 1.44 ± 0.27 KPa (liver). Viscosity: 0.98 ± 0.43 KPa (spleen) and 0.53 ± 0.21 KPa (liver)). Similar results were obtained for volunteers 2 and 3. (Volunteer2: Elasticity: 1.97 ± 0.50 KPa (spleen) and 1.42 ± 0.29 KPa (liver). Viscosity: 0.81 ± 0.53 KPa (spleen) and 0.44 ± 0.19 KPa (liver). Volunteer3: Elasticity: 2.46 ± 1.03 KPa (spleen) and 1.74 ± 0.52 KPa (liver). Viscosity: 1.10 ± 0.79 KPa (spleen) and 0.68 ± 0.37 KPa (liver)).
Conclusion
We have introduced Intenso, a novel sequence that enables 3D volumetric MRE of the liver in a single breath-hold and shown initial in-vivo results in three healthy volunteers matching expected anatomy (liver and spleen), as the data acquired is not degraded due to misalignment of different breath-holds. A clinical study is now warranted to evaluate the technique in patients with liver fibrosis and inflammation.Acknowledgements
This research recieved funding from European Union’s Horizon 2020 Research and Innovation Programme under grant agreement No 668039.In addition, this work was supported by the CRUK City of London Centre Award [C7893/A26233).
References
1. Sinkus, R, Lambert, S, Abd‐Elmoniem, KZ, et al. Rheological determinants for simultaneous staging of hepatic fibrosis and inflammation in patients with chronic liver disease. NMR in Biomedicine. 2018; 31:e3956.
2. Venkatesh, S.K., Yin, M. and Ehman, R.L. (2013), Magnetic resonance elastography of liver: Technique, analysis, and clinical applications. J. Magn. Reson. Imaging, 37: 544-555.
3. Garteiser P, Sahebjavaher RS, Ter Beek LC, Salcudean S, Vilgrain V, Van Beers BE, Sinkus R. Rapid acquisition of multifrequency, multislice and multidirectional MR elastography data with a fractionally encoded gradient echo sequence. NMR Biomed. 2013 Oct;26(10):1326-35. doi: 10.1002/nbm.2958. Epub 2013 May 28. PMID: 23712852.
4. Staeb D,Speier P, Reiter T , Klink T , Neubauer H ,Bley T A ,Wech T,Max Weng A, Kstler H. Restating MS-CAIPIRINHA as an In-plane Acceleration Problem: An Efficient Method for Integrating High Coverage Cardiac Perfusion MRI into Clinical Workflow. ISMRM 2015. Abstract #2686
5. Guenthner C, Sethi S, Troelstra M, Dokumaci AS, Sinkus R, Kozerke S. Ristretto MRE: A generalized multi-shot GRE-MRE sequence. NMR Biomed. 2019 May;32(5):e4049. doi: 10.1002/nbm.4049. Epub 2019 Jan 29. PMID: 30697827; PMCID: PMC6590281.
6. Breuer FA, Blaimer M, Heidemann RM, Mueller MF, Griswold MA, Jakob PM. Controlled aliasing in parallel imaging results in higher acceleration (CAIPIRINHA) for multi-slice imaging. Magn Reson Med. 2005 Mar;53(3):684-91. doi: 10.1002/mrm.20401. PMID: 15723404.
7. Guenthner C, Runge JH, Sinkus R, Kozerke S. Analysis and improvement of motion encoding in magnetic resonance elastography. NMR Biomed. 2018 May;31(5):e3908. doi: 10.1002/nbm.3908. Epub 2018 Mar 30. PMID: 29601114; PMCID: PMC6585970.
8. Runge JH, Hoelzl SH, Sudakova J, Dokumaci AS, Nelissen JL, Guenthner C, Lee J, Troelstra M, Fovargue D, Stoker J, Nederveen AJ, Nordsletten D, Sinkus R. A novel magnetic resonance elastography transducer concept based on a rotational eccentric mass: preliminary experiences with the gravitational transducer. Phys Med Biol. 2019 Feb 6;64(4):045007. doi: 10.1088/1361-6560/aaf9f8. PMID: 30566925.
9. Sinkus, R., Tanter, M., Catheline, S., Lorenzen, J., Kuhl, C., Sondermann, E. and Fink, M. (2005), Imaging anisotropic and viscous properties of breast tissue by magnetic resonance‐elastography. Magn. Reson. Med., 53: 372-387.
Figures