1875
Isotropic resolution volumetric liver T2 weighted imaging and T2 mapping using a navigator-gated radial stack-of-stars T2 prepared acquisition
Mark Zamskiy1, Dominik Weidlich1, Kilian Weiss2, Marcus Makowski1, Rickmer Braren1, and Dimitrios Karampinos1
1Department of Diagnostic and Interventional Radiology, School of Medicine, Technical University of Munich, Munich, Germany, 2Philips Healthcare, Hamburg, Germany
1Department of Diagnostic and Interventional Radiology, School of Medicine, Technical University of Munich, Munich, Germany, 2Philips Healthcare, Hamburg, Germany
Synopsis
Volumetric liver T2-weighted imaging and T2 mapping is of a high clinical significance but still remains very challenging due to motion in this region. The present work introduces a novel T2-prepared radial stack-of-stars (SoS) gradient echo sequence, which can be combined with a DIXON readout for achieving isotropic resolution fat-suppressed T2-weighted images. Moreover, the combination of the T2-prepared SoS acquisition with different T2-preparations enables T2 mapping in navigator-gated scans.
Purpose:
Volumetric T2-weighted imaging is of high clinical interest in the characterization of focal liver lesions and T2 mapping has shown promising results in the staging of liver fibrosis1,2. 3D T2-weighted imaging and T2 mapping of the liver remains however challenged by the strong sensitivity of the region to respiratory motion and has been frequently performed using 2D acquisitions and breath-hold scans3. Radial stack-of-stars (SoS) acquisitions allow volumetric coverage and have been gaining popularity for liver imaging due to their inherent robustness to motion and self-navigation capabilities4-7. Recent work presented a framework for inversion-recovery-prepared radial stack-of-stars imaging and demonstrated its feasibility for volumetric T1 mapping in the liver during free breathing8. A T2-prepared radial SoS gradient echo technique would be an excellent candidate for the acquisition of isotropic resolution volumetric liver T2-weighted images applicable in the characterization of focal lesions and diffuse liver disease. The acquisition of multiple T2 weightings also offers the possibility for volumetric T2 mapping in the liver. The present work aims to propose a navigator-gated radial stack-of-stars acquisition using an adiabatic T2-preparation for isotropic resolution volumetric liver T2-weighted imaging and T2 mapping.Methods:
Pulse Sequence:An adiabatic T2-preparation module9, 10 was combined with a radial SoS turbo field echo (TFE) readout (Fig.1). The 3D SoS readout allows volumetric imaging and is less motion-sensitive in comparison to other trajectories. By changing the duration of the gaps between the segments of the adiabatic BIR-4 RF-pulse in the T2-preparation, different T2 weightings can be achieved. After each excitation pulse in the TFE train, either a single echo (to increase SNR, Fig.1A) or multiple echoes (to enable water-fat decomposition of the signal using a multi-point Dixon approach, Fig.1B) can be acquired.
Simulations:
To characterize the T2-weighting profile for different B1 and B0 offsets, Bloch simulations of the magnetization after BIR-4 RF pulse were performed for ΔB0 = -250:10:250 Hz and for B1 = 60:5:120 %.
In vivo - Fat suppressed liver imaging:
The proposed T2-prepared TFE sequence was combined with navigator gating and was acquired with a 2-point DIXON readout in a healthy volunteer with the following sequence parameters: FOV: 400x400x200 mm3, acquisition voxel size: 3 mm isotropic, TR/TE1/TE2 = 3.8/1.11/2.2 ms, TFE angle = 8° and shot duration: 550 ms, effective duration of the T2 preparation: 15 ms, nominal scan time: 1m44s. For water-fat-decomposition, the vendor’s implementation was used11.
In vivo - liver relaxometry:
The proposed T2-prepared navigator-gated TFE sequence was acquired with a single echo readout in two healthy volunteers and the following sequence parameters: FOV: 400x400x200 mm3, acquisition voxel size: 3 mm isotropic, TR/TE = 2.7/1.11 ms, TFE angle = 8° and shot duration: 550 ms, effective duration of the T2-preparation: 10 / 15 / 20 ms, nominal scan time: 5m8s. For comparison, the vendor’s multi-echo GraSE T2 mapping sequence with the following parameters (FOV: 400x400x200 mm3, acquisition voxel size: 3 mm isotropic, 8 echoes, TR = 1641 ms, TEs = 16 ms to 64 ms in 8 ms steps) acquired in 2m24s using respiratory triggering and a multi-TE single voxel STEAM MRS (TR/TM = 1200/16 ms, VOI = 15x15x15mm3, 1 average per TE, TEs = 10 ms to 55 ms in 5 ms steps) acquired in an 11s breath-hold were performed in the liver tissue. T2 values from the imaging sequences were calculated based on a voxel-wise mono-exponential fitting of the data. The processing of the MRS data was performed with in-house software and included zero-order phasing, gaussian apodization and frequency alignment. Peak area quantification was performed considering ten fat peaks and one water peak and jointly incorporating the measurement of all TEs with individual T2s for each peak.
Results:
Fig.2 shows that the adiabatic T2-preparation has a high tolerance to B1 inhomogeneities and has a stable performance for B0 inhomogeneities between -170 and 170 Hz.Fig.3 shows the ability to effectively suppress the fat signal and acquire 3D isotropic resolution volumetric fat-suppressed T2-weighted images when the T2-preparation is combined with a DIXON readout.
In Fig.4 the obtained T2 maps for GraSE and the T2-prepared TFE are shown as well as the acquired spectroscopy sequence. Assuming that the MRS sequence can be considered as a gold standard sequence, the GraSE leads to an overestimation of 76 % whereas the T2-prepared TFE only underestimates T2 by 18%. The GraSE T2 maps show overall higher sensitivity to motion.
Discussion & Conclusion:
The present work introduces a novel T2-prepared radial SoS gradient echo sequence and demonstrates its applications: fat-suppressed volumetric T2-weighted imaging in the combination with a 2-echo DIXON readout and the possibility for 3D isotropic resolution T2-mapping in the liver.The current work also has several limitations: the signal behaviour depends on the T1 values of the measured tissue and sequence parameters, such as flip angle, the delay time and the time of the acquisition of the k-space center and therefore further validation is required regarding the accuracy of the estimated liver T2 values.
Nevertheless, the proposed sequence might be a promising acquisition framework for volumetric isotropic resolution T2-weighted imaging and T2 mapping in the liver.
Acknowledgements
The present work was support by the TUM International Graduate School of Science and Engineering (TUM-ICL Joint Academy of Doctoral Studies), the German Research Foundation (DFG-SFB824/A9) and Philips Healthcare.References
- Hoffman, D.H., Ayoola, A., Nickel, D. et al. T1 mapping, T2 mapping and MR elastography of the liver for detection and staging of liver fibrosis. Abdom Radiol 45, 692–700 (2020). https://doi.org/10.1007/s00261-019-02382-9
- Guimaraes AR, Siqueira L, Uppal R, Alford J, Fuchs BC, Yamada S, Tanabe K, Chung RT, Lauwers G, Chew ML, Boland GW, Sahani DV, Vangel M, Hahn PF, Caravan P. T2 relaxation time is related to liver fibrosis severity. Quant Imaging Med Surg. 2016 Apr;6(2):103-14. doi: 10.21037/qims.2016.03.02. PMID: 27190762; PMCID: PMC4858462.
- Fahlenkamp UL, Ziegeler K, Adams LC, Böker SM, Engel G, Makowski MR. Native T1 mapping for assessment of the perilesional zone in metastases and benign lesions of the liver. Sci Rep. 2020 Jul 30;10(1):12889. doi: 10.1038/s41598-020-69819-w. PMID: 32733016; PMCID: PMC7393097.
- Chandarana H, Block TK, Rosenkrantz AB, Lim RP, Kim D, Mossa DJ, Babb JS, Kiefer B, Lee VS. Free-breathing radial 3D fat-suppressed T1-weighted gradient echo sequence: a viable alternative for contrast-enhanced liver imaging in patients unable to suspend respiration. Investigative radiology2011;46(10):648-653.
- Benkert T, Mugler JP, 3rd, Rigie DS, Sodickson DK, Chandarana H, Block KT. Hybrid T2 - and T1 -weighted radial acquisition for free-breathing abdominal examination. Magn Reson Med 2018;80(5):1935-1948.
- Benkert T, Feng L, Sodickson DK, Chandarana H, Block KT. Free-breathing volumetric fat/water separation by combining radial sampling, compressed sensing, and parallel imaging. Magn Reson Med 2017;78(2):565-576.
- Feng L, Grimm R, Block KT, Chandarana H, Kim S, Xu J, Axel L, Sodickson DK, Otazo R. Golden-angle radial sparse parallel MRI: combination of compressed sensing, parallel imaging, and golden-angle radial sampling for fast and flexible dynamic volumetric MRI. Magn Reson Med 2014;72(3):707-717.
- Feng L, Block KT, Benkert T, Tian Y, Liu C, Liu F, Fayad Z, Yang Y. Stack-of-Stars Inversion-Recovery MRI for Free-Breathing T1 Mapping and IR-PreparedFat/Water Separation, Proc ISMRM 2020, p 1015
- Weidlich D, Schlaeger S, Kooijman H, Börnert P, Kirschke JS, Rummeny EJ, Haase A, Karampinos DC. T2 mapping with magnetization-prepared 3D TSE based on a modified BIR-4 T2 preparation. NMR Biomed. 2017 Nov;30(11). doi: 10.1002/nbm.3773. Epub 2017 Aug 4. PMID: 28777496.
- Klupp E, Weidlich D, Schlaeger S, Baum T, Cervantes B, Deschauer M, Kooijman H, Rummeny EJ, Zimmer C, Kirschke JS, Karampinos DC. B1-insensitive T2 mapping of healthy thigh muscles using a T2-prepared 3D TSE sequence. PLoS One. 2017 Feb 14;12(2):e0171337. doi: 10.1371/journal.pone.0171337. PMID: 28196133; PMCID: PMC5308846.
- Dixon WT. Simple proton spectroscopic imaging. Radiology. 1984 Oct;153(1):189-94. doi: 10.1148/radiology.153.1.6089263. PMID: 6089263.
Figures
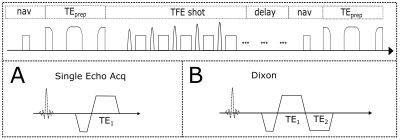
Fig.1. T2-prepared TFE SoS navigator-gated sequence design. The T2-preparation consists of modified BIR-4 pulse and is followed by a TFE SoS shot and a delay before the next navigator. The TFE SoS readout can be performed with (A) single echo acquisition and (B) two echo DIXON readout.
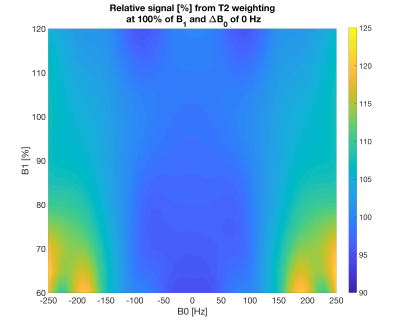
Fig.2. Simulated relative signal map from T2-weighting at 100% B1 and 0Hz ΔB0 for the effective echo time of the preparation of 15 ms. Similar T2 weightings are observed for a wide range of B1 values and B0 inhomogeneities between -170 and 170 Hz.
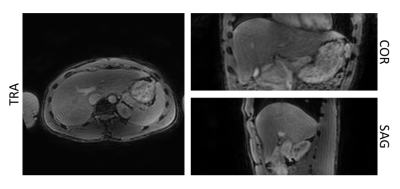
Fig.3. Separated T2-weighted water images obtained from the T2-prepared TFE acquisition with DIXON readout for with an effective echo time of the T2-preparation of 15ms. The fat signal is effectively suppressed, and the resulting isotropic resolution T2-weighted image allows reformatting in all 3 planes.
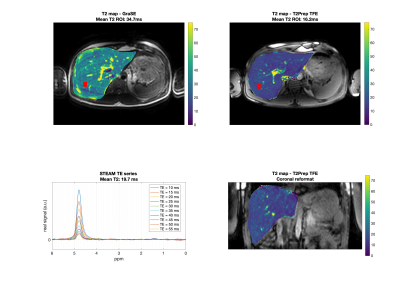
Fig.4. First row: Axial T2 maps obtained from GraSE and T2-prepared TFE. The red box represents the MRS voxel. Second row: MRS and the T2-prepared TFE coronal reformatted T2 map. Mean ROI value represents the mean value within the MRS acquisition voxel. T2 values from the imaging sequences deviate by 76 % (GraSE) and 18% (T2-prepared TFE) from MRS. Artifacts due to motion are visible in the GraSE but not in the T2-prepared TFE sequence.