1863
Effect of subject-specific T1 values for arterial spin labelling on cerebral blood flow in mild stroke patients1Centre for Clinical Brain Sciences, University of Edinburgh, Edinburgh, United Kingdom, 2UK DRI at the University of Edinburgh, Edinburgh, United Kingdom, 3Hurvitz Brain Sciences Research Program, Sunnybrook Health Sciences Centre, Toronto, ON, Canada, 4Department of Medical Biophysics, University of Toronto, Toronto, ON, Canada
Synopsis
Accurate cerebral blood flow (CBF) quantification using arterial spin labelling (ASL) relies on physiological and MR parameters. Longitudinal relaxation time (T1) of blood, which depends on haematocrit, can be a factor in some patient groups. We determined subject-specific T1 using the DESPOT-1 HIFI method in a mild stroke cohort, calculating CBF using nominal and subject-specific values. CBF calculated with subject-specific T1 values was lower in grey and higher in white matter, though there was not a proportional bias. CBF was lower in patients with higher disease burden. Subject-specific T1 values can reduce variance, potentially improving CBF quantification in clinical ASL.
Introduction
Small vessel disease (SVD) is an important factor in many strokes and dementia1. Cerebral blood flow (CBF) and cerebrovascular reactivity are important measurements to help further our understanding of SVD. Arterial spin labelling yields CBF maps and is increasingly common for clinical MRI2. Accurate quantification depends upon various factors, including the longitudinal relaxation time (T1). A constant literature value is typically used3. However, as T1 of blood (T1b) depends on haematocrit4-6 several different values have been used7. Subject-specific T1 values, from quantitative T1 maps, may better account for inter-subject variability8, especially in patients where variation could be greater7,9,10. We assessed the effect of calculating CBF with subject-specific T1 values in mild stroke patients.Methods
We recruited patients with recent mild ischaemic strokes after informed consent for an on-going study acquiring clinical, demographic, and imaging variables11. We performed MRI scans on a 3T Prisma scanner (Siemens Healthcare, Germany) including a standard neurovascular imaging protocol (T1-w, T2-w, FLAIR and DTI), multi-inversion time (TI) 3D pseudo-continuous ASL (pCASL) sequence (12 equally spaced TIs=500-3030ms, repetition time (TR)/echo time (TE)=4350/20.98ms with 4 background suppression pulses7, bolus duration=1800ms, 32-channel head coil), two inversion recovery (IR) spoiled gradient echo sequences (TR=1040, 1940 ms, TE=1.82 ms, TI=600, 1500 ms, flip angle (FA)=5°) and three spoiled gradient echo sequences with variable flip angle (TR/TE=5.4/1.82 ms, FA=2°, 5°, 12°). We acquired T1 maps with voxelwise correction for flip angle error $$$(K=FA_{true}/FA_{nom})$$$ using the DESPOT1-HIFI method12 and derived parametric maps13. We manually selected five voxels from the superior sagittal sinus to estimate a mean T1b11,14.We processed the ASL data to obtain whole brain CBF using a 1-compartment model with a nominal labelling efficiency of 0.615 and standard or subject-specific T1 values through BASIL16, 17. We segmented white matter hyperintensity (WMH) using a semi-automatic approach and manually delineated stroke masks11. We excluded WMH and stroke voxels before calculating the mean white and grey matter CBF for each approach. Differences between the two CBF values were assessed with Bland-Altman plots. We also applied a linear mixed model to predict CBF accounting for calculation using nominal or estimated T1 values (1/0), tissue type (WM/GM=1/0), age, diagnosed/not diagnosed hypertension (1/0), total Fazekas score18 and the interaction of calculation method with tissue type using the lme4 and emmeans libraries in the R software package v.3.6.3.
Results
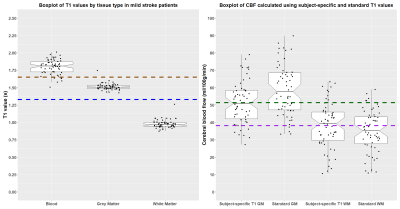
We analysed data from 59 patients with masks in the superior sagittal sinus (mean age: 68, range: 51-86, 40% female). One patient was excluded due to intravascular signal artefact. The T1b was 1.80±0.11 s, tissue T1 was 1.51±0.05 s in GM and 0.98±0.06 s in WM (Figure 1A). Mean CBF was lower in GM and higher in WM when calculated with subject-specific than nominal T1 values (GM: 51.4±12.4 vs 59.0±14.7, WM: 38.2±13.2 vs 35.1±11.9 ml/100g/min, Figure 1B). Bland-Altman plots showed no proportional bias in WM or GM CBF when calculated with estimated T1 values (Figure 2). A linear mixed model showed an interaction between tissue type and processing with subject-specific T1 values. CBF was lower in GM (B=7.6, CI: 9.29, 5.90, p<0.001) and higher in WM (B=-3.07, CI: -1.38, -4.77, p<0.001) when calculated with estimated T1 values (Table 1). Additionally, CBF tended to be lower in patients with higher disease burden (B=-2.67, CI: -5.51, 0.17, p=0.071). On average CBF was lower in older patients and higher in patients with diagnosed hypertension, however the confidence intervals were broad.Discussion
T1 values in tissue and blood varied across the cohort, calculating CBF with estimated rather than nominal T1 values led to lower GM and higher WM CBF. We found mean blood, GM and WM T1 were comparable to previously reported values13,19-21. Applying subject-specific T1 values may improve the accuracy of CBF estimates in stroke patients by better controlling for inter-patient variability and disease severity, especially as T1 may vary pathologically in WMH22. We also showed patients with higher disease burden had lower CBF in grey and white matter after excluding WMH consistent with previous findings in SVD23 and Alzheimer’s disease24. Age and hypertension are key vascular risk factors for SVD25. Although we found CBF decreased with age the association was weak, with broad confidence intervals. SVD burden and poor lifestyle factors at younger ages may impact vascular health affecting associations with age26,27. Similarly, while hypertension has been associated with reduced CBF28, intensive blood pressure lowering regimens may increase CBF in treated/untreated groups29, potentially contributing to the broad confidence intervals. Future work will aim to explore these associations in greater detail by incorporating additional markers of disease burden, including perivascular spaces, and examine the influence of partial volume correction around WMH.Conclusion
Acquiring quantitative T1 maps in mild stroke patients is feasible, and differentially affects grey and white matter CBF values. Incorporating subject-specific T1 values when calculating CBF in stroke patients may better account for inter-subject variability7, potentially enhancing sensitivity to subtle associations. However, as quantitative T1 imaging requires specialist processing optimal approaches for deriving representative T1 values or atlases may be worth exploring for patient populations.Acknowledgements
We acknowledge the assistance of Siemens Healthcare GmbH and Dr Josef Pfeuffer for providing the Advanced 3D ASL work-in-progress sequence. Funding is gratefully acknowledged from the Fondation Leducq (ref no. 16 CVD 05), European Union Horizon 2020 project No. 666881, ‘SVDs@Target’ and the Scottish Funding Council through the Scottish Imaging Network, A Platform for Scientific Excellence (SINAPSE) Collaboration and their Postdoctoral and Early Career Researcher Exchanges scheme. We also thank the participants, Edinburgh Imaging physicists, radiographers and professional support staff for their involvement in this work.References
1. Wardlaw JM, Smith C, Dichgans M. Small vessel disease: mechanisms and clinical implications. The Lancet Neurology 2019;18:684-696.
2. Grade M, Hernandez Tamames JA, Pizzini FB, Achten E, Golay X, Smits M. A neuroradiologist's guide to arterial spin labeling MRI in clinical practice. Neuroradiology 2015;57:1181-1202.
3. Alsop DC, Detre JA, Golay X, et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med 2015;73:102-116.
4. Brooks RA, Di Chiro G. Magnetic resonance imaging of stationary blood: a review. Med Phys 1987;14:903-913.
5. Bryant RG, Marill K, Blackmore C, Francis C. Magnetic relaxation in blood and blood clots. Magn Reson Med 1990;13:133-144.
6. Silvennoinen MJ, Kettunen MI, Kauppinen RA. Effects of hematocrit and oxygen saturation level on blood spin-lattice relaxation. Magn Reson Med 2003;49:568-571.
7. Fan AP, Jahanian H, Holdsworth SJ, Zaharchuk G. Comparison of cerebral blood flow measurement with [15O]-water positron emission tomography and arterial spin labeling magnetic resonance imaging: A systematic review. J Cereb Blood Flow Metab 2016;36:842-861.
8. Lu H, Clingman C, Golay X, van Zijl PC. Determining the longitudinal relaxation time (T1) of blood at 3.0 Tesla. Magn Reson Med 2004;52:679-682.
9. Pollock JM, Tan H, Kraft RA, Whitlow CT, Burdette JH, Maldjian JA. Arterial spin-labeled MR perfusion imaging: clinical applications. Magn Reson Imaging Clin N Am 2009;17:315-338.
10. Hales PW, Kawadler JM, Aylett SE, Kirkham FJ, Clark CA. Arterial spin labeling characterization of cerebral perfusion during normal maturation from late childhood into adulthood: normal 'reference range' values and their use in clinical studies. J Cereb Blood Flow Metab 2014;34:776-784.
11. Clancy U, Garcia DJ, Stringer MS, et al. Rationale and design of a longitudinal study of cerebral small vessel diseases, clinical and imaging outcomes in patients presenting with mild ischaemic stroke: Mild Stroke Study 3. European Stroke Journal;0:2396987320929617.
12. Deoni SC. High-resolution T1 mapping of the brain at 3T with driven equilibrium single pulse observation of T1 with high-speed incorporation of RF field inhomogeneities (DESPOT1-HIFI). J Magn Reson Imaging 2007;26:1106-1111.
13. Thrippleton MJ, Blair GW, Valdes-Hernandez MC, et al. MRI Relaxometry for Quantitative Analysis of USPIO Uptake in Cerebral Small Vessel Disease. Int J Mol Sci 2019;20.
14. Heye AK, Thrippleton MJ, Armitage PA, et al. Tracer kinetic modelling for DCE-MRI quantification of subtle blood--brain barrier permeability. Neuroimage 2016;125:446-455.
15. Vidorreta M, Wang Z, Rodriguez I, Pastor MA, Detre JA, Fernandez-Seara MA. Comparison of 2D and 3D single-shot ASL perfusion fMRI sequences. Neuroimage 2013;66:662-671.
16. Chappell MA, Groves AR, Whitcher B, Woolrich MW. Variational Bayesian Inference for a Nonlinear Forward Model. IEEE Transactions on Signal Processing 2009;57:223-236.
17. Chappell MA, Groves AR, MacIntosh BJ, Donahue MJ, Jezzard P, Woolrich MW. Partial volume correction of multiple inversion time arterial spin labeling MRI data. Magn Reson Med 2011;65:1173-1183.
18. Fazekas F, Niederkorn K, Schmidt R, et al. White matter signal abnormalities in normal individuals: correlation with carotid ultrasonography, cerebral blood flow measurements, and cerebrovascular risk factors. Stroke 1988;19:1285-1288.
19. Marques JP, Kober T, Krueger G, van der Zwaag W, Van de Moortele PF, Gruetter R. MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. Neuroimage 2010;49:1271-1281.
20. Stikov N, Boudreau M, Levesque IR, Tardif CL, Barral JK, Pike GB. On the accuracy of T1 mapping: searching for common ground. Magn Reson Med 2015;73:514-522.
21. Wu WC, Jain V, Li C, et al. In vivo venous blood T1 measurement using inversion recovery true-FISP in children and adults. Magn Reson Med 2010;64:1140-1147.
22. De Guio F, Vignaud A, Chabriat H, Jouvent E. Different types of white matter hyperintensities in CADASIL: Insights from 7-Tesla MRI. J Cereb Blood Flow Metab 2018;38:1654-1663.
23. Stewart CR, Stringer MS, Shi Y, Thrippleton MJ, Wardlaw JM. Associations between white matter hyperintensity burden, cerebral blood flow and transit time in small vessel disease: an updated meta-analysis. medRxiv 2020:2020.2010.2006.20207373.
24. Benedictus MR, Binnewijzend MAA, Kuijer JPA, et al. Brain volume and white matter hyperintensities as determinants of cerebral blood flow in Alzheimer's disease. Neurobiol Aging 2014;35:2665-2670.
25. Staals J, Makin SD, Doubal FN, Dennis MS, Wardlaw JM. Stroke subtype, vascular risk factors, and total MRI brain small-vessel disease burden. Neurology 2014;83:1228-1234.
26. Backhouse EV, McHutchison CA, Cvoro V, Shenkin SD, Wardlaw JM. Early Life Risk Factors for Stroke and Cognitive Impairment. Current Epidemiology Reports 2015;2:172-179.
27. McHutchison CA, Chappell FM, Makin S, Shuler K, Wardlaw JM, Cvoro V. Stability of Estimated Premorbid Cognitive Ability over Time after Minor Stroke and Its Relationship with Post-Stroke Cognitive Ability. Brain Sci 2019;9.
28. Wang T, Li Y, Guo X, et al. Reduced perfusion in normal-appearing white matter in mild to moderate hypertension as revealed by 3D pseudocontinuous arterial spin labeling. J Magn Reson Imaging 2016;43:635-643.
29. Tryambake D, He J, Firbank MJ, O'Brien JT, Blamire AM, Ford GA. Intensive blood pressure lowering increases cerebral blood flow in older subjects with hypertension. Hypertension 2013;61:1309-1315.
Figures