1848
A separate RF Neck Coil for Arterial Spin Labeling at 7T MRI1Bioengineering, University of Pittsburgh, Pittsburgh, PA, United States, 2University of Pittsburgh, Pittsburgh, PA, United States
Synopsis
A dedicated labeling coil for arterial spin labeling (ASL) technique can alleviate the challenges at 7T MRI, in this work we propose a separate 16-channel RF neck coil for transmit only. Finite-difference time-domain (FDTD) simulations and RF shimming have demonstrated the feasibility of this design to produce a homogeneous B1+ fields in the labeling region (left and right common carotid arteries) while minimizing SAR to within safe limits.
Introduction
Arterial Spin Labeling (ASL) technique has become the main noninvasive technique in clinical and research protocols to assess cerebral perfusion and quantify cerebral blood flow (CBF) by magnetically labeling the inflowing blood and utilizing it as an endogenous contrast agent (1). Intrinsically, ASL Perfusion weighted images have relatively low signal-to-noise ratio (SNR), therefore, improving SNR is essential to obtain more precise CBF estimation (2). Technology developments at 7 tesla (T) MRI have progressed to be cleared as the standard field strength for ultra-high field (UHF) MR for human imaging , offering the advantages of a significant increase in SNR and a longer blood longitudinal relaxation time (T1). ASL at UHF still suffers from some technical challenges such as B1+ inhomogeneity, B1+ field deterioration below the brain area, B0 inhomogeneity that can impact the labeling efficiency, magnetic transfer (MT) effects, and most importantly higher global/local specific absorption rate (SAR) (3,4). To overcome these challenges, we propose a separate RF transmit only (Tx) multi channels volume neck coil that enhances B1+ distribution, intensity, and tagging efficiency in the labeling region of interest (left and right common carotid arteries) while keeping SAR within regulated limits.Methods
The RF coil used in this project employs the Tic-Tac-Toe (TTT) coil design, many recent studies have presented the performance of this design in different body parts (5–7). In this work, four TTT panels are positioned around the neck (three anterior and one posterior), each panel consists of four channels or excitation points configuring four rows of elements along the Z-axis and totaling of 16 channels, as shown in figure (1, a).Computational simulations using finite-difference time-domain (FDTD) method integrated with a transmission line model are utilized to numerically calculate the electromagnetic components of the RF fields in each voxel of a detailed high-resolution anatomical model (Duke). 3D configuration of the design is constructed using Matlab (Natick, MA) as shown in figure (1, b), the head coil is included in the FDTD grid as a shield to mimic the actual environment during labeling. Coil bench testing was performed using vector network analyzer (VNA) on a spherical phantom to validate the simulation outputs and S-parameters. Upon validation, the simulation output fields were optimized by manipulating the amplitude weights and phase shifts of the current waveform that feeds each individual channel which allowed viewing different B1+ fields distribution and power deposition values, the advantage of having 16 channels (degree of freedom=32) expanded the model RF shimming capability.
Results
Tuning/matching validation of the coil performance is shown in figure (2), calculated S-parameters from the simulation are in agreement with the experimental data measured on a VNA. Figure (2) also shows the experimental B1+ map on a spherical phantom after applying a particular shim case (phases, amplitudes) exported from the simulation.As illustrated in figure (3), Results of RF shimming have concluded four different implementable cases, B1+ values are scaled to ‘µTesla for 1W’ and SAR values are scaled to ‘W/kg per 1W averaged over 10 g of tissue’. To determine homogeneity, Coefficient of variation (CoV) was calculated in a mask that was contoured around ROI. Multislice tool enabled the localization of SAR ‘hot spots’ and B1+ field continuity.
Discussions and Conclusions
A separate neck coil dedicated for labeling evolves the feasibility of ASL implementation at 7T. Results demonstrated the ability of this coil to produce homogenous B1+ field in the tagging plane while minimizing SAR to an acceptable range. The flexibility of altering the trade-offs between B1+ fields and SAR give rise to achieve the highest labeling efficiency percentage for different labeling schemes. Additionally, tagging at the neck area significantly minimize MT effects.Acknowledgements
This work was supported by the National Institutes of Health under award numbers: R01MH111265, R01AG063525, T32MH119168 , This research was also supported in part by the University of Pittsburgh Center for Research Computing through the resources provided.References
References
1. Grade M, Hernandez Tamames JA, Pizzini FB, Achten E, Golay X, Smits M. A neuroradiologist’s guide to arterial spin labeling MRI in clinical practice. Neuroradiology. 2015 Dec;57(12):1181–202.
2. Ivanov D, Gardumi A, Haast RAM, Pfeuffer J, Poser BA, Uludağ K. Comparison of 3T and 7T ASL techniques for concurrent functional perfusion and BOLD studies. NeuroImage. 2017 Aug 1;156:363–76.
3. Bandettini PA, Bowtell R, Jezzard P, Turner R. Ultrahigh field systems and applications at 7 T and beyond: Progress, pitfalls, and potential: UHF Systems and Applications at 7 T and Beyond. Magn Reson Med. 2012 Feb;67(2):317–21.
4. Pohmann R, Speck O, Scheffler K. Signal-to-noise ratio and MR tissue parameters in human brain imaging at 3, 7, and 9.4 tesla using current receive coil arrays: SNR at 9.4T. Magn Reson Med. 2016 Feb;75(2):801–9.
5. Santini T, Kim J, Wood S, Krishnamurthy N, Farhat N, Maciel C, et al. A new RF transmit coil for foot and ankle imaging at 7T MRI. Magn Reson Imaging. 2018 Jan;45:1–6.
6. Kim J, Santini T, Bae KT, Krishnamurthy N, Zhao Y, Zhao T, et al. Development of a 7 T RF coil system for breast imaging. NMR Biomed [Internet]. 2017 Jan [cited 2019 Nov 17];30(1). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5943082/
7. Santini T, Zhao Y, Wood S, Krishnamurthy N, Kim J, Farhat N, et al. In-vivo and numerical analysis of the eigenmodes produced by a multi-level Tic-Tac-Toe head transmit array for 7 Tesla MRI. PLoS ONE [Internet]. 2018 Nov 27 [cited 2019 Nov 17];13(11). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6258503/
Figures
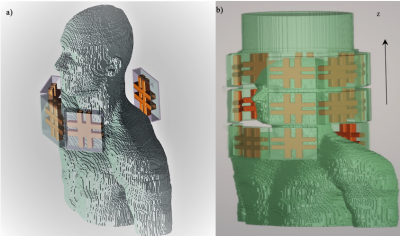
Figure (1), In a) The arrangement of the neck coil alone around the neck of Duke model. In b) Configuration of the single system to be simulated.
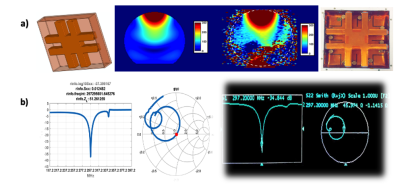
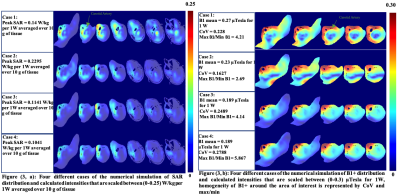
Figure (3, a): Four different cases of the numerical simulation of SAR distribution and calculated intensities that are scaled between (0-0.25) W/kg per 1W averaged over 10 g of tissue, Figure (3, b): Four different cases of the numerical simulation of B1+ distribution and calculated intensities that are scaled between (0-0.3) µTesla for 1W, homogeneity of B1+ around the area of interest is represented by CoV and max/min