1845
Reproducibility and Validation of Water Permeability in Human Brain using Magnetization Transfer based ASL at 7T1Department of Electrical and Computer Engineering, Auburn University, Auburn, AL, United States, 2Auburn University MRI Research Center, Auburn University, Auburn, AL, United States
Synopsis
Blood-brain barrier (BBB) is crucial to prevent brain tissue from circulating toxins while allowing the delivery of nutrients from intravascular space to the central nervous system (CNS). Compromised BBB can result in brain dysfunction and degeneration. Development of reproducible non-invasive techniques to assess BBB permeability is of particular interest. Previously we had demonstrated a non-invasive technique to estimate BBB permeability using magnetization transfer (MT) effect on labeled arterial spins at 7T. In this work, we demonstrate the reproducibility of the technique. The feasibility was evaluated in healthy subjects at baseline and after caffeine challenge.
Introduction
Blood-brain barrier (BBB) is a tightly regulated biochemical and anatomical system that supplies brain cells with nutrients, protects the brain from harmful compounds and regulates ion balance 1,2. It acts as a selective diffusion barrier and is compromised in pathological conditions such as inflammatory disease, neoplasm etc. 3. Previously, we demonstrated a non-invasive technique to assess BBB permeability using magnetization transfer (MT) effect on ASL perfusion signal 4. The technique relies on the fact that, when additional MT pulses are applied to saturate brain macromolecules during ASL perfusion measurement, the vascular water and tissue water experience very different MT effect. 5,6. This allows us to quantify perfusion, water extraction fraction and permeability surface area product in the brain. In this work, we demonstrate the reproducibility of the developed technique. Furthermore we tested the sensitivity of the technique to measure change in water extraction fraction and perfusion due to caffeine intake.Methods
All the experiments were performed with Siemens 7T Magnetom with 32 channel head coil. Using QUIPSS II FAIR ASL technique, perfusion (f in mL/100g/min), water extraction fraction (E), permeability surface area product (PS in mL/100g/min) and magnetization transfer ratio (MTR) were calculated as described previously 4. Slice selective and non-selective inversions were achieved with 8 ms hyperbolic secant adiabatic pulse. Other imaging parameters were: FOV= 256 mm, slice thickness=8 mm, TR=2 s, TE=1.39 s, TI1=0.8 s, TI2= 1.8 s, flip angle=10o. Double saturation with pulse duration=2.56 ms was used to cut off the blood bolus at TI1. MT pulse with offset frequency=500 Hz and duration=16.64 ms was used to saturate the macromolecules. All the images were acquired in this order: tag (MT on)/control (MT on)/tag (MT off)/control (MT off). Test-retest reliability was determined in 4 subjects, who were scanned twice on the same day. Caffeine challenge was performed in 3 subjects to validate the method. The participants were asked to refrain from all kind of caffeine intake for 7 hours prior to the study and the studies were performed approximately same time in the morning. After initial (pre-caffeine) scans, subjects were removed from the scanner and given 200 mg caffeine pills. Subjects were imaged again approximately 20 minutes after the caffeine intake.Results
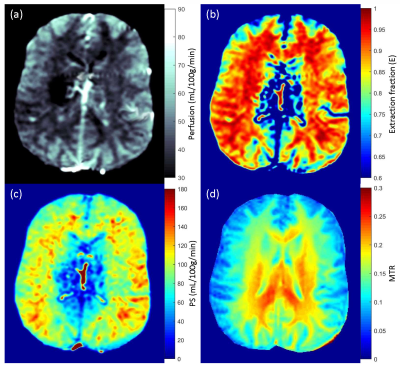
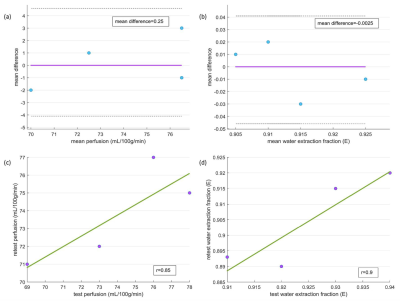
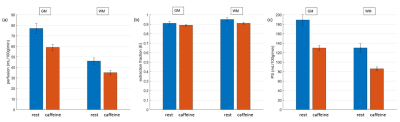
Perfusion, water extraction fraction, permeability surface area product and magnetization transfer ratio maps are shown in Fig. 1. Bland-Altman plots for perfusion and water extraction fraction (gray matter ROI) show very good agreement for test-retest (Fig. 2a and 2b). Mean differences for perfusion and water extraction fraction were 0.25 and -0.0025 respectively for the test-retest analysis. Perfusion (r = 0.85) and water extraction fraction (r = 0.9) were highly correlated in the test-retest experiments (Fig. 2c and 2d). Fig. 3 shows the comparison of perfusion, water extraction fraction and permeability surface area products before and after caffeine intake. Perfusion was decreased from 77±5 to 59±3 mL/100g/min in gray matter and 46±3 to 35±2 mL/100g/min in white matter following caffeine intake (p=0.04). Extraction fraction was reduced from 0.91±0.02 to 0.89±0.01 in gray matter and 0.95±0.02 to 0.91±0.01 in white matter following caffeine intake (p=0.02). Permeability surface area product also decreased from 189±11 to 130±5 mL/100g/min in gray matter and 130±9 to 86±4 mL/100g/min in white matter following caffeine intake (p=0.009).Discussion
In this study we have demonstrated the reproducibility of a technique that utilizes magnetization transfer effect on perfusion signal to assess BBB permeability. We also validated the result with caffeine challenge, which is in good agreement with previous reports 7. This technique can be very useful in investigating the BBB integrity in healthy as well as pathological conditions and non-invasive nature will allow for repeated studies.Acknowledgements
No acknowledgement found.References
1. Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev 2005;57(2):173-185.
2. Persidsky Y, Ramirez SH, Haorah J, et al. Blood-brain barrier: structural components and function under physiologic and pathologic conditions. J Neuroimmune Pharmacol 2006;1(3):223-236.
3. Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis 2004;16(1):1-13.
4. Mahmud SZ, Denney TS, Bashir A. Measurement of Blood-Brain Barrier Permeability in Human Brain using Magnetization Transfer Effect at 7T. In Proceedings of 28th Annual Meeting of ISMRM, 2020. p 1749.
5. Balaban RS, Ceckler TL. Magnetization transfer contrast in magnetic resonance imaging. Magn Reson Q 1992;8(2):116-137.
6. Dousset V, Degreze P, Mieze S, et al. Magnetization transfer on in vitro circulating blood: Implications for time-of-flight MR angiography. Jmri-J Magn Reson Im 1995;5(6):786-788.
7. Wengler K, Bangiyev L, Canli T, et al. 3D MRI of whole-brain water permeability with intrinsic diffusivity encoding of arterial labeled spin (IDEALS). Neuroimage 2019;189:401-414.
Figures